2-AminoethanolCAS# 141-43-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 141-43-5 | SDF | Download SDF |
| PubChem ID | 700 | Appearance | Powder |
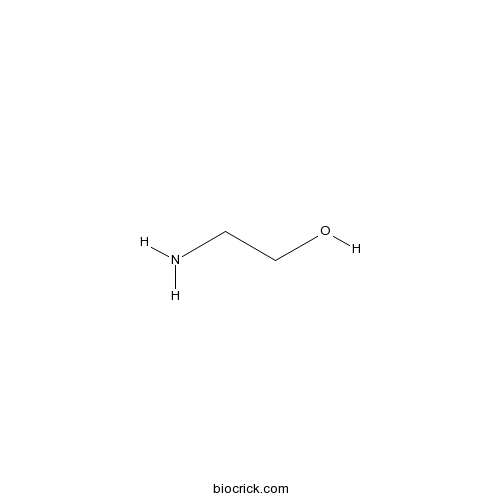
| Formula | C2H7NO | M.Wt | 61.08 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-aminoethanol | ||
| SMILES | C(CO)N | ||
| Standard InChIKey | HZAXFHJVJLSVMW-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2-Aminoethanol (ethanolamine) can inhibit the GABA degrading enzyme GABA aminotransferase (GABA-T), it is a possible neuromodulator in the pigeon optic tectum. Strengthening of the antioxidant mechanisms by plant pretreatment with 2-aminoethanol could be helpful in the development of a broad tolerance to adverse stress conditions. |
| Targets | GABA Receptor |
| In vitro | Effect of 2-aminoethanol pretreatment on the antioxidant enzyme activity in Zea mays under oxidative stress[Reference: WebLink]Russian Journal of Plant Physiology, 2011,58 (1):45-50.Exposure of plants to stress may result in liberation of 2-Aminoethanol as an inducer of alarm reaction that activates cellular resistance and tolerance mechanisms. |
| In vivo | 2-Aminoethanol as a possible neuromodulator in the pigeon optic tectum.[Pubmed: 7145226]Neurosci Lett. 1982 Sep 20;32(1):53-8.
|
| Kinase Assay | Effect of 2-aminoethanol on the synthesis, binding, uptake and metabolism of GABA[Pubmed: 6320073]Neurosci Lett. 1983 Dec 11;42(3):293-7.2-Aminoethanol (ethanolamine) was studied for effects on neurochemical assays for GABA synthesis, receptor binding, uptake and metabolism in rat brain preparations. |
| Structure Identification | J. Phys. Chem. A, 2002, 106 (4), pp 668–679Hydrogen Bonding in Monomers and Dimers of 2-Aminoethanol[Reference: WebLink]
|

2-Aminoethanol Dilution Calculator

2-Aminoethanol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 16.372 mL | 81.8599 mL | 163.7197 mL | 327.4394 mL | 409.2993 mL |
| 5 mM | 3.2744 mL | 16.372 mL | 32.7439 mL | 65.4879 mL | 81.8599 mL |
| 10 mM | 1.6372 mL | 8.186 mL | 16.372 mL | 32.7439 mL | 40.9299 mL |
| 50 mM | 0.3274 mL | 1.6372 mL | 3.2744 mL | 6.5488 mL | 8.186 mL |
| 100 mM | 0.1637 mL | 0.8186 mL | 1.6372 mL | 3.2744 mL | 4.093 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ricinoleic acid
Catalog No.:BCC8248
CAS No.:141-22-0
- Nerylacetate
Catalog No.:BCN3802
CAS No.:141-12-8
- Benzo-15-crown 5-ether
Catalog No.:BCC8849
CAS No.:14098-44-3
- Benzo-18-crown-6 ether
Catalog No.:BCC8850
CAS No.:14098-24-9
- (2R,3S)-Chlorpheg
Catalog No.:BCC6805
CAS No.:140924-23-8
- 6-Hydroxy-5,6-dehydrosugiol
Catalog No.:BCN3127
CAS No.:140923-35-9
- 4-Chloro-D-phenylalanine
Catalog No.:BCC2637
CAS No.:14091-08-8
- Aminopotentidine
Catalog No.:BCC6761
CAS No.:140873-26-3
- ICI 215,001 hydrochloride
Catalog No.:BCC5688
CAS No.:140850-02-8
- 1-Hydroxymethyl-beta-carboline glucoside
Catalog No.:BCN7026
CAS No.:1408311-12-5
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- PF 06465469
Catalog No.:BCC6268
CAS No.:1407966-77-1
- Malonic acid
Catalog No.:BCN8534
CAS No.:141-82-2
- 2-Thiouracil
Catalog No.:BCC4752
CAS No.:141-90-2
- Frangulin B
Catalog No.:BCC8175
CAS No.:14101-04-3
- Gama-Tocotrienol
Catalog No.:BCN3720
CAS No.:14101-61-2
- PACOCF3
Catalog No.:BCC7074
CAS No.:141022-99-3
- 1-Hydroxy-2,3,4,7-tetramethoxyxanthone
Catalog No.:BCN6506
CAS No.:14103-09-4
- Xanomeline oxalate
Catalog No.:BCC4146
CAS No.:141064-23-5
- JNK-IN-8
Catalog No.:BCC1673
CAS No.:1410880-22-6
- 12-Hydroxydodecanoic Acid
Catalog No.:BCN8405
CAS No.:505-95-3
- TRAP-6
Catalog No.:BCC3957
CAS No.:141136-83-6
- Epiguajadial B
Catalog No.:BCN4075
CAS No.:1411629-26-9
- 2-(Dimethylaminomethyl)-2-propanol
Catalog No.:BCN1774
CAS No.:14123-48-9
2-Aminoethanol as a possible neuromodulator in the pigeon optic tectum.[Pubmed:7145226]
Neurosci Lett. 1982 Sep 20;32(1):53-8.
A mass fragmentographic method was used for determination of low molecular weight compounds in perfusates collected in vivo in the pigeon optic tectum by a push-pull cannula technique. 2-Aminoethanol (ethanolamine) could be collected under resting conditions (5.6 +/- 0.09 pmol/min). Electrical stimulation of optic nerve induced a 2.3-fold increase of the tectal ethanolamine outflow whereas that of GABA was not affected. Ethanolamine applied iontophoretically to tectal neurons did not influence their spontaneous discharge; however, their glutamate-induced excitation as well as the GABA-induced depression were enhanced if ethanolamine was applied simultaneously. It is suggested that optic nerve stimulation exerts a neuromodulatory effect on tectal neurons.
Effect of 2-aminoethanol on the synthesis, binding, uptake and metabolism of GABA.[Pubmed:6320073]
Neurosci Lett. 1983 Dec 11;42(3):293-7.
2-Aminoethanol (ethanolamine) was studied for effects on neurochemical assays for GABA synthesis, receptor binding, uptake and metabolism in rat brain preparations. The effects of ethanolamine were compared with those of ethanolamine O-sulphate (EOS), an inhibitor of GABA degradation. Furthermore, the effect of both compounds was compared on GABA metabolism in rat brain in vivo. In vitro, ethanolamine and EOS had no significant effect on the GABA synthesizing enzyme glutamic decarboxylase (GAD) and GABA uptake, but both drugs proved virtually equipotent to inhibit the GABA degrading enzyme GABA aminotransferase (GABA-T). EOS was a relatively potent inhibitor of GABA receptor binding, whereas ethanolamine was not effective in this regard. Following systemic administration in rats, 50% inhibition of GABA-T in the brain was achieved by 500 mg/kg ethanolamine or 2000 mg/kg EOS, respectively. As a consequence of GABA-T inhibition, GABA levels increased significantly. GAD activity remained unchanged after both treatments. The present results suggest that the recently reported enhancement of functional effects of GABA by ethanolamine may relate, at least in part, to the inhibitory effect of the compound on GABA catabolism.


