PACOCF3CPLA2 and iPLA2 inhibitor CAS# 141022-99-3 |
- Raltitrexed
Catalog No.:BCC4457
CAS No.:112887-68-0
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

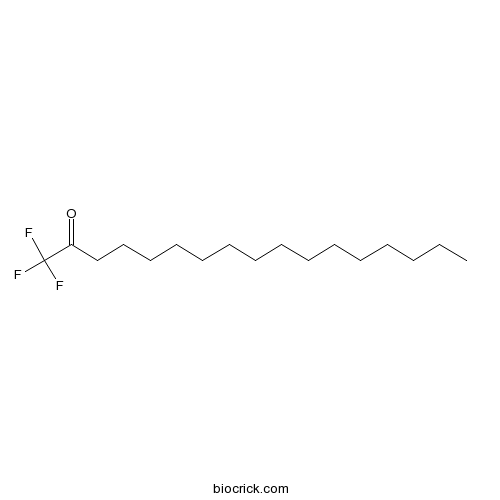
| Cas No. | 141022-99-3 | SDF | Download SDF |
| PubChem ID | 4670 | Appearance | Powder |
| Formula | C17H31F3O | M.Wt | 308.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Palmityl trifluoromethyl ketone | ||
| Solubility | Soluble to 25 mM in DMSO and to 50 mM in ethanol | ||
| Chemical Name | 1,1,1-trifluoroheptadecan-2-one | ||
| SMILES | CCCCCCCCCCCCCCCC(=O)C(F)(F)F | ||
| Standard InChIKey | MAHYXYTYTLCTQD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H31F3O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(21)17(18,19)20/h2-15H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Phospholipase A2 inhibitor. Can also alter Ca2+ signaling in renal tubular cells. |

PACOCF3 Dilution Calculator

PACOCF3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2422 mL | 16.2111 mL | 32.4223 mL | 64.8445 mL | 81.0557 mL |
| 5 mM | 0.6484 mL | 3.2422 mL | 6.4845 mL | 12.9689 mL | 16.2111 mL |
| 10 mM | 0.3242 mL | 1.6211 mL | 3.2422 mL | 6.4845 mL | 8.1056 mL |
| 50 mM | 0.0648 mL | 0.3242 mL | 0.6484 mL | 1.2969 mL | 1.6211 mL |
| 100 mM | 0.0324 mL | 0.1621 mL | 0.3242 mL | 0.6484 mL | 0.8106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PACOCF3 is a inhibitor of both Ca2+-dependent cytosolic cPLA2 and Ca2+-independent phospholipases A2 iPLA2 with IC50 values of 45 µM and 3.8 µM [1,2], which is an innovate and potential candidate drug for inflammation, atherosclerosis, diabetes mellitus, therapeutic shock, cancer therapyetc [3,4,5,6]. It has been reported that PACOCF3 can inhibit PLA2 and reduce the inflammatory response. PACOCF3 also can stimulate insulin release at basal glucose levels (2 mmol/l). Application of PACOCF3 can inhibit endogenous arachidonic acid generation which significantly decreased the amplitude of the insulin secretory response to 20 mmol/l glucose [7,8]. PACOCF3 have a dual protective role in diabetes which could minimize β-cell dysfunction while maintaining insulin secretory output through enhancing endogenous arachidonic acid levels.
References:
1. Murakami M et al.Emerging roles of secreted phospholipase A2 enzymes: The 3rd edition.Biochimie. 2014 Sep 16. pii: S0300-9084(14)00252-1.
2. Quach ND et al. Secretory phospholipase A2 enzymes as pharmacological targets for treatment of disease. Biochem Pharmacol. 2014 Aug 15;90(4):338-48.
3. Chalimoniuk M. Secretory phospholipase A2 and its role in oxidative stress and inflammation]. Postepy Biochem. 2012;58(2):204-8.
4. Persaud SJ.et al. The role of arachidonic acid and its metabolites in insulin secretion from human islets of langerhans. Diabetes. 2007 Jan;56(1):197-203.
5. Khakpour H et al. Lipoprotein-associated phospholipase A2: an independent predictor of cardiovascular risk and a novel target for immunomodulation therapy. Cardiol Rev. 2009 Sep-Oct;17(5):222-9.
6. Narendra Sharath Chandra JN1 et al. Chemistry and structural evaluation of different phospholipase A2 inhibitors in arachidonic acid pathway mediated inflammation and snake venom toxicity. Curr Top Med Chem. 2007;7(8):787-800.
7. Packard CJ. Lipoprotein-associated phospholipase A2 as a biomarker of coronary heart disease and a therapeutic target. Curr Opin Cardiol. 2009 Jul;24(4):358-63.
8. Lucas R et al. Synthesis and enzyme inhibitory activities of a series of lipidic diamine and aminoalcohol derivatives on cytosolic and secretory phospholipases A2. Bioorg Med Chem Lett. 2000 Feb 7;10(3):285-8.
- Gama-Tocotrienol
Catalog No.:BCN3720
CAS No.:14101-61-2
- Frangulin B
Catalog No.:BCC8175
CAS No.:14101-04-3
- 2-Thiouracil
Catalog No.:BCC4752
CAS No.:141-90-2
- Malonic acid
Catalog No.:BCN8534
CAS No.:141-82-2
- 2-Aminoethanol
Catalog No.:BCN1756
CAS No.:141-43-5
- Ricinoleic acid
Catalog No.:BCC8248
CAS No.:141-22-0
- Nerylacetate
Catalog No.:BCN3802
CAS No.:141-12-8
- Benzo-15-crown 5-ether
Catalog No.:BCC8849
CAS No.:14098-44-3
- Benzo-18-crown-6 ether
Catalog No.:BCC8850
CAS No.:14098-24-9
- (2R,3S)-Chlorpheg
Catalog No.:BCC6805
CAS No.:140924-23-8
- 6-Hydroxy-5,6-dehydrosugiol
Catalog No.:BCN3127
CAS No.:140923-35-9
- 4-Chloro-D-phenylalanine
Catalog No.:BCC2637
CAS No.:14091-08-8
- 1-Hydroxy-2,3,4,7-tetramethoxyxanthone
Catalog No.:BCN6506
CAS No.:14103-09-4
- Xanomeline oxalate
Catalog No.:BCC4146
CAS No.:141064-23-5
- JNK-IN-8
Catalog No.:BCC1673
CAS No.:1410880-22-6
- 12-Hydroxydodecanoic Acid
Catalog No.:BCN8405
CAS No.:505-95-3
- TRAP-6
Catalog No.:BCC3957
CAS No.:141136-83-6
- Epiguajadial B
Catalog No.:BCN4075
CAS No.:1411629-26-9
- 2-(Dimethylaminomethyl)-2-propanol
Catalog No.:BCN1774
CAS No.:14123-48-9
- Btk inhibitor 1
Catalog No.:BCC4238
CAS No.:1412418-47-3
- Asenapine hydrochloride
Catalog No.:BCC1371
CAS No.:1412458-61-7
- QS-21
Catalog No.:BCC8243
CAS No.:141256-04-4
- PEPA
Catalog No.:BCC5951
CAS No.:141286-78-4
- Orobanchyl acetate
Catalog No.:BCN7779
CAS No.:1413843-71-6
Dual action of palmitoyl trifluoromethyl ketone (PACOCF3) on Ca2+ signaling: activation of extracellular Ca2+ influx and alteration of ATP- and bradykinin-induced Ca2+ responses in Madin Darby canine kidney cells.[Pubmed:11097381]
Arch Toxicol. 2000 Oct;74(8):447-51.
The effect of the phospholipase A2 inhibitor palmitoyl trifluoromethyl ketone (PACOCF3) on Ca2+ signaling in Madin Darby canine kidney (MDCK) cells was examined using fura-2 as the fluorescent Ca2+ indicator. At a concentration of 20 microM, PACOCF3 did not change basal cytosolic free calcium concentrations ([Ca2+]i), but at concentrations of 50-250 microM PACOCF3 induced an increase in [Ca2+]i by activating extracellular Ca2+ entry which was partly suppressed by 50 microM La3+. The effect of PACOCF3 was abolished by removal of extracellular Ca2+. PACOCF3 (10 microM) enhanced both the peak value and the area under the curve of the [Ca2+]i increase induced by 10 microM ATP and 1 microM bradykinin by potentiating extracellular Ca2+ influx without affecting internal Ca2+ release. Several other phospholipase A2 inhibitors had no effect on basal [Ca2+]i or agonist-induced [Ca2+]i increases. Collectively, the results suggest that PACOCF3 alters Ca2+ signaling in renal tubular cells in a manner independent of phospholipase A2 inhibition.
Irreversible inhibition of Ca(2+)-independent phospholipase A2 by methyl arachidonyl fluorophosphonate.[Pubmed:8695655]
Biochim Biophys Acta. 1996 Jul 12;1302(1):55-60.
Methyl arachidonyl fluorophosphonate (MAFP) has been recently reported to be a selective, active-site directed, irreversible inhibitor of the Group IV 85 kDa cytosolic phospholipase A2 (cPLA2). We have now shown that this compound also potently inhibits the Ca(2+)-independent cytosolic phospholipase A2 (iPLA2). MAFP inhibited iPLA2 in a concentration-dependent manner with half-maximal inhibition observed at 0.5 microM after a 5 min preincubation at 40 degrees C. This inhibition was not reversed upon extensive dilution of the enzyme into the assay mixture. Preincubation of iPLA2 with MAFP resulted in a linear, time-dependent inactivation of enzyme activity, and the enzyme was protected from inactivation by the reversible inhibitor PACOCF3. The ability of MAFP to inhibit the iPLA2 suggests that this enzyme proceeds through an acyl-enzyme intermediate as has been proposed for the cPLA2. Further testing indicated that MAFP did not inhibit the arachidonoyl-CoA synthetase, CoA-dependent acyltransferase, or CoA-independent transacylase activities from P388D1 cells. Thus, MAFP is not a general inhibitor for enzymes which act on arachidonoyl substrates. Instead, the inhibitor appears to show some selectivity for PLA2, although it does not discriminate between cPLA2 and iPLA2. Particular caution must be exercised to distinguish these activities if this inhibitor is used in intact cells.
Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones.[Pubmed:7814408]
J Biol Chem. 1995 Jan 6;270(1):445-50.
A novel Ca(2+)-independent phospholipase A2 (PLA2) has recently been purified from the murine macrophage-like cell line P388D1 (Ackermann, E. J., Kempner, E. S., and Dennis, E. A. (1994) J. Biol. Chem. 269, 9227-9233). This enzyme is now shown to be inhibited by palmitoyl trifluoromethyl ketone (PACOCF3), arachidonyl trifluoromethyl ketone (AACOCF3), and a bromoenol lactone (BEL). Both PACOCF3 and AACOCF3 were found to inhibit the macrophage PLA2 in a concentration-dependent manner. PACOCF3 was found to be approximately 4-fold more potent than AACOCF3, with IC50 values of 3.8 microM (0.0075 mol fraction) and 15 microM (0.028 mol fraction), respectively. Reaction progress curves in the presence of either inhibitor were found to be linear, and the PACOCF3.PLA2 complex rapidly dissociated upon dilution. BEL was also found to inhibit the macrophage PLA2 in a concentration-dependent manner, with half-maximal inhibition observed at 60 nM after a 5-min preincubation at 40 degrees C. Inhibition was not reversed after extensive dilution of the enzyme into assay buffer. Treatment of the PLA2 with BEL resulted in a linear, time-dependent inactivation of activity, and the rate of this inactivation was diminished in the presence of PACOCF3. In addition, PLA2 treated with [3H]BEL resulted in the covalent labeling of a major band at M(r) 80,000. Inactivation of the PLA2 by 5,5'-dithiobis(2-nitrobenzoic acid) prior to treatment with [3H]BEL resulted in the near complete lack of labeling consistent with covalent irreversible suicide inhibition of the enzyme. The labeling of a M(r) 80,000 band rather than a M(r) 40,000 band upon treatment with [3H]BEL distinguishes the macrophage Ca(2+)-independent PLA2 from a previously identified myocardial Ca(2+)-independent PLA2 and provides strong evidence that the M(r) 80,000 protein is the catalytic subunit.


