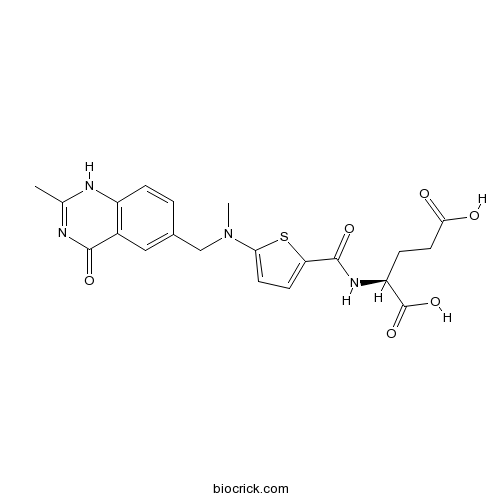
RaltitrexedCAS# 112887-68-0 |
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- Elvitegravir (GS-9137)
Catalog No.:BCC2134
CAS No.:697761-98-1
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
- HIV-1 integrase inhibitor 2
Catalog No.:BCC1619
CAS No.:957890-42-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112887-68-0 | SDF | Download SDF |
| PubChem ID | 104758 | Appearance | Powder |
| Formula | C21H22N4O6S | M.Wt | 458.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ZD1694; D1694; ICI-D1694 | ||
| Solubility | DMSO : ≥ 29 mg/mL (63.25 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S)-2-[[5-[methyl-[(2-methyl-4-oxo-1H-quinazolin-6-yl)methyl]amino]thiophene-2-carbonyl]amino]pentanedioic acid | ||
| SMILES | CC1=NC(=O)C2=C(N1)C=CC(=C2)CN(C)C3=CC=C(S3)C(=O)NC(CCC(=O)O)C(=O)O | ||
| Standard InChIKey | IVTVGDXNLFLDRM-HNNXBMFYSA-N | ||
| Standard InChI | InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Raltitrexed is an inhibitor of thymidylate synthase and an antimetabolite drug used for cancer treatment.In Vitro:Raltitrexed inhibits HepG2 proliferation by arresting the cell cycle at G0/G1, and the cell cycle is mediated via downregulation of cyclin A and CDK2[1]. Raltitrexed (0.1, 0.5, 2.5 μg/mL) decreases the viability of SGC7901 cells in a dose- and time-dependent manner. Raltitrexed (0.5 μg/mL) shows typical apoptotic morphology, including nuclear shrinkage, fragmentation, chromatin condensation and apoptotic bodies in SGC7901 cells. Raltitrexed blocks the cell cycle at the G0/G1 phase, decreases in the mitochondrial membrane potential. Raltitrexed also increases the level of ROS, induces caspase-3-dependent apoptosis via activation of the mitochondria, and increases TS protein and mRNA expression levels[3]. Raltitrexed (1.5 nM) reduces the number of GM00637 cells, selectively induces gene conversions, but does not affect DSB-induced HR or NHEJ[4].In Vivo:Raltitrexed (0, 5, 10, 11.5, 13.5, 15 mg/kg b/w, i.p.) increases the rates of resorbed embryos and growth retardation of murine model of NTDs in a dose dependent manner. Raltitrexed (11.5 mg/kg b/w) maximally inhibits the thymidylate synthase (TS) activity in embryonic tissue, decreases dTMP levels and while increases dUMP levels[2]. References: | |||||

Raltitrexed Dilution Calculator

Raltitrexed Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1811 mL | 10.9054 mL | 21.8107 mL | 43.6215 mL | 54.5268 mL |
| 5 mM | 0.4362 mL | 2.1811 mL | 4.3621 mL | 8.7243 mL | 10.9054 mL |
| 10 mM | 0.2181 mL | 1.0905 mL | 2.1811 mL | 4.3621 mL | 5.4527 mL |
| 50 mM | 0.0436 mL | 0.2181 mL | 0.4362 mL | 0.8724 mL | 1.0905 mL |
| 100 mM | 0.0218 mL | 0.1091 mL | 0.2181 mL | 0.4362 mL | 0.5453 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Raltitrexed (Tomudex, TDX, ZD 1694) is an antimetabolite drug used in cancer chemotherapy. It is an inhibitor of thymidylate synthase.
- Mosapride Citrate
Catalog No.:BCC1065
CAS No.:112885-42-4
- Mosapride
Catalog No.:BCC4078
CAS No.:112885-41-3
- Fmoc-2-Nal-OH
Catalog No.:BCC3292
CAS No.:112883-43-9
- Fmoc-N-Me-Nle-OH
Catalog No.:BCC3299
CAS No.:112883-42-8
- Fmoc-D-Nle-OH
Catalog No.:BCC3300
CAS No.:112883-41-7
- Fmoc-D-Met-OH
Catalog No.:BCC3532
CAS No.:112883-40-6
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- 24R-Calcipotriol
Catalog No.:BCC1304
CAS No.:112827-99-3
- Methyllycaconitine citrate
Catalog No.:BCC6897
CAS No.:112825-05-5
- Dynole 34-2
Catalog No.:BCC7891
CAS No.:1128165-88-7
- 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCC8464
CAS No.:112811-72-0
- BzATP triethylammonium salt
Catalog No.:BCC7643
CAS No.:112898-15-4
- Boc-D-Asp(OcHex)-OH
Catalog No.:BCC3372
CAS No.:112898-18-7
- HU 211
Catalog No.:BCC5946
CAS No.:112924-45-5
- PD128907 HCl
Catalog No.:BCC4469
CAS No.:112960-16-4
- Calcipotriol
Catalog No.:BCC1444
CAS No.:112965-21-6
- Tokinolide B
Catalog No.:BCN2753
CAS No.:112966-16-2
- IM-12
Catalog No.:BCC5487
CAS No.:1129669-05-1
- Estradiol diproppionate
Catalog No.:BCC8960
CAS No.:113-38-2
- Chlorprothixene
Catalog No.:BCC3753
CAS No.:113-59-7
- Chlorpheniramine Maleate
Catalog No.:BCC4526
CAS No.:113-92-8
- Potassium benzylpenicillin
Catalog No.:BCC9126
CAS No.:113-98-4
- VU 0155069
Catalog No.:BCC7715
CAS No.:1130067-06-9
Raltitrexed Inhibits HepG2 Cell Proliferation via G0/G1 Cell Cycle Arrest.[Pubmed:27098147]
Oncol Res. 2016;23(5):237-48.
Raltitrexed (RTX) is an antimetabolite drug used as a chemotherapeutic agent for treating colorectal cancer, malignant mesothelioma, and gastric cancer. The antitumor capacity of RTX is attributed to its inhibitory activity on thymidylate synthase (TS), a key enzyme in the synthesis of DNA precursors. The current study is aimed at investigating the potential antitumor effects of RTX in liver cancer. Using the HepG2 cell line as an in vitro model of liver cancer, we evaluated the effects of RTX on cell proliferation employing both a WST-8 assay and a clone formation efficiency assay. In addition, we monitored the ultrastructure changes of HepG2 cells in response to RTX with transmission electric microscopy. To investigate the mechanism underlying the regulation of cell proliferation by RTX, we analyzed cell cycle using cell flow cytometry. Moreover, real-time PCR and Western blot analyses were conducted to examine expression levels of cell cycle regulatory proteins cyclin A and cyclin-dependent kinase 2 (CDK2), as well as their mediators tumor suppressor genes p53 and p16. Our results demonstrate that RTX inhibits HepG2 proliferation by arresting the cell cycle at G0/G1. This cell cycle arrest function was mediated via downregulation of cyclin A and CDK2. The observed elevated expression of p53 and p16 by RTX may contribute to the reduction of cyclin A/CDK2. Our study indicates that RTX could serve as a potential chemotherapeutic agent in the treatment of hepatocellular carcinoma.
Hepatic artery infusion with raltitrexed or 5-fluorouracil for colorectal cancer liver metastasis.[Pubmed:28293087]
World J Gastroenterol. 2017 Feb 28;23(8):1406-1411.
AIM: To evaluate the efficiency and safety of hepatic artery infusion chemotherapy (HAIC) using Raltitrexed or 5-fluorouracil for colorectal cancer (CRC) liver metastasis (CRCLM). METHODS: A retrospective analysis of patients with unresectable CRCLM who failed systemic chemotherapy and were subsequently treated with HAIC at our institute from May 2013 to April 2015 was performed. A total of 24 patients were treated with 5-fluorouracil, and 18 patients were treated with Raltitrexed. RESULTS: The median survival time (MST) from diagnosis of CRC was 40.8 mo in the oxaliplatin plus Raltitrexed (TOMOX) arm and 33.5 mo in the oxaliplatin plus 5-fluorouracil (FOLFOX) arm (P = 0.802). MST from first HAIC was 20.6 mo in the TOMOX arm and 15.4 mo in the FOLFOX arm (P = 0.734). Median progression-free survival (PFS) from first HAIC was 4.9 mo and 6.6 mo, respectively, in the TOMOX arm and FOLFOX arm (P = 0.215). Leukopenia (P = 0.026) was more common in the FOLFOX arm, and hepatic disorder (P = 0.039) was more common in the TOMOX arm. There were no treatment-related deaths in the TOMOX arm and one treatment-related death in the FOLFOX arm. Analysis of prognostic factors indicated that response to HAIC was a significant factor related to survival. CONCLUSION: No significant difference in survival was observed between the TOMOX and FOLFOX arms. HAIC treatment with either TOMOX or FOLFOX was demonstrated as an efficient and safe alternative choice.
Structural Analysis of Thymidylate Synthase from Kaposi's Sarcoma-Associated Herpesvirus with the Anticancer Drug Raltitrexed.[Pubmed:27936107]
PLoS One. 2016 Dec 9;11(12):e0168019.
Kaposi's sarcoma-associated herpesvirus (KSHV) is a highly infectious human herpesvirus that causes Kaposi's sarcoma. KSHV encodes functional thymidylate synthase, which is a target for anticancer drugs such as Raltitrexed or 5-fluorouracil. Thymidylate synthase catalyzes the conversion of 2'-deoxyuridine-5'-monophosphate (dUMP) to thymidine-5'-monophosphate (dTMP) using 5,10-methylenetetrahydrofolate (mTHF) as a co-substrate. The crystal structures of thymidylate synthase from KSHV (apo), complexes with dUMP (binary), and complexes with both dUMP and Raltitrexed (ternary) were determined at 1.7 A, 2.0 A, and 2.4 A, respectively. While the ternary complex structures of human thymidylate synthase and E. coli thymidylate synthase had a closed conformation, the ternary complex structure of KSHV thymidylate synthase was observed in an open conformation, similar to that of rat thymidylate synthase. The complex structures of KSHV thymidylate synthase did not have a covalent bond between the sulfhydryl group of Cys219 and C6 atom of dUMP, unlike the human thymidylate synthase. The catalytic Cys residue demonstrated a dual conformation in the apo structure, and its sulfhydryl group was oriented toward the C6 atom of dUMP with no covalent bond upon ligand binding in the complex structures. These structural data provide the potential use of antifolates such as Raltitrexed as a viral induced anticancer drug and structural basis to design drugs for targeting the thymidylate synthase of KSHV.
Raltitrexed plus oxaliplatin-based transarterial chemoembolization in patients with unresectable hepatocellular carcinoma.[Pubmed:27145327]
Anticancer Drugs. 2016 Aug;27(7):689-94.
Raltitrexed has shown efficacy and safety in many tumor types; however, the clinical data on the treatment of hepatocellular carcinoma is rare. In this report, we aim to assess the efficacy and safety of Raltitrexed plus oxaliplatin (OXA)-based transarterial chemoembolization (TACE) in patients with unresectable hepatocellular carcinoma (uHCC). Patients with uHCC were recruited from multi-centers in China and assigned randomly to Raltitrexed+OXA-based (n=76), fluorouracil+OXA-based (n=76), and doxorubicin+OXA-based (n=75) TACE treatment. The primary end point was overall survival (OS). Tumor response was assessed using response evaluation criteria in solid tumors (RECIST), modified response evaluation criteria in solid tumors (mRECIST), and European Association for the Study of the Liver criteria (EASL). Safety and toxicity were evaluated using the National Cancer Institute Common Toxicity Criteria. The Raltitrexed group showed a better disease control rate evaluated using RECIST (Raltitrexed vs. fluorouracil vs. doxorubicin: 96.1 vs. 84.2 vs. 86.7%, P=0.05) and a better overall response rate on the basis of mRECIST (67.1 vs. 47.4 vs. 50.7%, P=0.03) and EASL (67.1 vs. 47.4 vs. 49.3%, P=0.02). The median OS and median progression-free survival (PFS) were higher in the Raltitrexed group (median OS: 13.4 vs. 9.6 vs. 8.5 months; median PFS: 6.7 vs 4.9 vs 4.6 months). The most common toxicities included elevated aspartate aminotransferase (78.9 vs. 86.8 vs. 81.3%) and abdominal nonspecific pain (68.4 vs. 81.6 vs. 78.7%). No significant differences were found in the overall number of patients who experienced any toxicity. Raltitrexed plus OXA-based TACE suggested a safe and efficacious regimen in uHCC patients. The results warrant further clinical investigation.


