Mosapride Citrate5-HT receptor agonist CAS# 112885-42-4 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- 7-Xylosyltaxol
Catalog No.:BCN5341
CAS No.:90332-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112885-42-4 | SDF | Download SDF |
| PubChem ID | 119583 | Appearance | Powder |
| Formula | C27H33ClFN3O10 | M.Wt | 614.02 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Mosapride (citrate); AS-4370; TAK-370 | ||
| Solubility | DMSO : 100 mg/mL (162.86 mM; Need ultrasonic) | ||
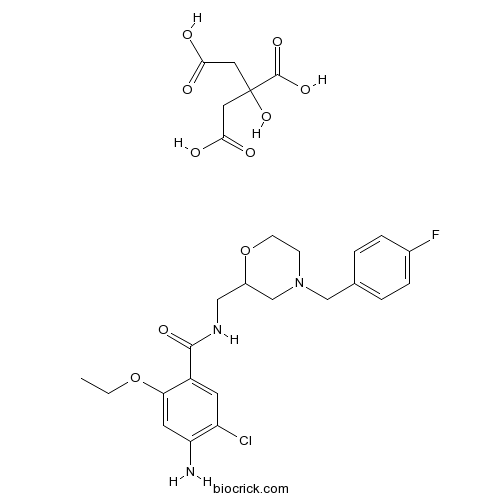
| Chemical Name | 4-amino-5-chloro-2-ethoxy-N-[[4-[(4-fluorophenyl)methyl]morpholin-2-yl]methyl]benzamide;2-hydroxypropane-1,2,3-tricarboxylic acid | ||
| SMILES | CCOC1=CC(=C(C=C1C(=O)NCC2CN(CCO2)CC3=CC=C(C=C3)F)Cl)N.C(C(=O)O)C(CC(=O)O)(C(=O)O)O | ||
| Standard InChIKey | HUZTYZBFZKRPFG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H25ClFN3O3.C6H8O7/c1-2-28-20-10-19(24)18(22)9-17(20)21(27)25-11-16-13-26(7-8-29-16)12-14-3-5-15(23)6-4-14;7-3(8)1-6(13,5(11)12)2-4(9)10/h3-6,9-10,16H,2,7-8,11-13,24H2,1H3,(H,25,27);13H,1-2H2,(H,7,8)(H,9,10)(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT4 receptor agonist and 5-HT3 receptor antagonist. Displays no activity at D2, α1, α2, 5-HT1 and 5-HT2 receptors. Gastroprokinetic agent; increases gastric emptying in rats and stimulates gastric motor actvity in conscious dogs. |

Mosapride Citrate Dilution Calculator

Mosapride Citrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6286 mL | 8.1431 mL | 16.2861 mL | 32.5722 mL | 40.7153 mL |
| 5 mM | 0.3257 mL | 1.6286 mL | 3.2572 mL | 6.5144 mL | 8.1431 mL |
| 10 mM | 0.1629 mL | 0.8143 mL | 1.6286 mL | 3.2572 mL | 4.0715 mL |
| 50 mM | 0.0326 mL | 0.1629 mL | 0.3257 mL | 0.6514 mL | 0.8143 mL |
| 100 mM | 0.0163 mL | 0.0814 mL | 0.1629 mL | 0.3257 mL | 0.4072 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
5-HT4 receptor agonist and 5-HT3 receptor antagonist. Displays no activity at D2, α1, α2, 5-HT1 and 5-HT2 receptors. Gastroprokinetic agent; increases gastric emptyin
- Mosapride
Catalog No.:BCC4078
CAS No.:112885-41-3
- Fmoc-2-Nal-OH
Catalog No.:BCC3292
CAS No.:112883-43-9
- Fmoc-N-Me-Nle-OH
Catalog No.:BCC3299
CAS No.:112883-42-8
- Fmoc-D-Nle-OH
Catalog No.:BCC3300
CAS No.:112883-41-7
- Fmoc-D-Met-OH
Catalog No.:BCC3532
CAS No.:112883-40-6
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- 24R-Calcipotriol
Catalog No.:BCC1304
CAS No.:112827-99-3
- Methyllycaconitine citrate
Catalog No.:BCC6897
CAS No.:112825-05-5
- Dynole 34-2
Catalog No.:BCC7891
CAS No.:1128165-88-7
- 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCC8464
CAS No.:112811-72-0
- 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid ethyl ester
Catalog No.:BCC8465
CAS No.:112811-71-9
- Raltitrexed
Catalog No.:BCC4457
CAS No.:112887-68-0
- BzATP triethylammonium salt
Catalog No.:BCC7643
CAS No.:112898-15-4
- Boc-D-Asp(OcHex)-OH
Catalog No.:BCC3372
CAS No.:112898-18-7
- HU 211
Catalog No.:BCC5946
CAS No.:112924-45-5
- PD128907 HCl
Catalog No.:BCC4469
CAS No.:112960-16-4
- Calcipotriol
Catalog No.:BCC1444
CAS No.:112965-21-6
- Tokinolide B
Catalog No.:BCN2753
CAS No.:112966-16-2
- IM-12
Catalog No.:BCC5487
CAS No.:1129669-05-1
- Estradiol diproppionate
Catalog No.:BCC8960
CAS No.:113-38-2
- Chlorprothixene
Catalog No.:BCC3753
CAS No.:113-59-7
- Chlorpheniramine Maleate
Catalog No.:BCC4526
CAS No.:113-92-8
- Potassium benzylpenicillin
Catalog No.:BCC9126
CAS No.:113-98-4
Measurement of Human Cytochrome P450 Enzyme Induction Based on Mesalazine and Mosapride Citrate Treatments Using a Luminescent Assay.[Pubmed:26336590]
Biomol Ther (Seoul). 2015 Sep;23(5):486-92.
Drug metabolism mostly occurs in the liver. Cytochrome P450 (CYP) is a drug-metabolizing enzyme that is responsible for many important drug metabolism reactions. Recently, the US FDA and EU EMA have suggested that CYP enzyme induction can be measured by both enzymatic activity and mRNA expression. However, these experiments are time-consuming and their inter-assay variability can lead to misinterpretations of the results. To resolve these problems and establish a more powerful method to measure CYP induction, we determined CYP induction by using luminescent assay. Luminescent CYP assays link CYP enzyme activity to firefly luciferase luminescence technology. In this study, we measured the induction of CYP isozymes (1A2, 2B6, 2C9, and 3A4) in cryopreserved human hepatocytes (HMC424, 478, and 493) using a luminometer. We then examined the potential induction abilities (unknown so far) of mesalazine, a drug for colitis, and Mosapride Citrate, which is used as an antispasmodic drug. The results showed that mesalazine promotes CYP2B6 and 3A4 activities, while Mosapride Citrate promotes CYP1A2, 2B6, and 3A4 activities. Luminescent CYP assays offer rapid and safe advantages over LC-MS/MS and qRT-PCR methods. Furthermore, luminescent CYP assays decrease the interference between the optical properties of the test compound and the CYP substrates. Therefore, luminescent CYP assays are less labor intensive, rapid, and can be used as robust tools for high-throughput CYP screening during early drug discovery.
Mosapride citrate increases postprandial glucagon-like peptide-1, insulin, and gene expression of sweet taste receptors.[Pubmed:25008428]
Dig Dis Sci. 2015 Feb;60(2):345-53.
BACKGROUND AND AIM: Mosapride Citrate-a prokinetic agent-improves hemoglobin A1c levels in diabetic patients; however, the underlying mechanism is unclear. We aimed to clarify this mechanism. METHODS: Preprandial and postprandial (90 min after a meal) blood was obtained from 12 healthy men, and serum insulin and plasma active glucagon-like peptide-1 concentrations were measured. Measurements were also taken after the administration of 5 mg of Mosapride Citrate three times per day after every meal for 14 days. In addition, C57BL/6 mice were permitted free access to water containing 0.04 % domperidone (D group) or 0.02 % Mosapride Citrate (M group) for 2 weeks (four mice per group). T1r2 (taste receptor, type 1, member 2), T1r3, and Gnat3 (guanine nucleotide-binding protein, alpha transducing 3) mRNA expression levels of the stomach, duodenum, and proximal and mid-jejunum were evaluated. RESULTS: In human subjects, postprandial plasma active glucagon-like peptide-1 and serum insulin concentrations after administration of Mosapride Citrate were significantly higher than those pre-administration (4.8 +/- 2.2 pmol/L, 45.6 +/- 41.6 muIU/mL, and 3.7 +/- 1.2 pmol/L, 34.1 +/- 28.4 muIU/mL, respectively). The mouse expression levels of T1r2 and Gnat3 in the proximal jejunum and mid-jejunum in the M group (4.1 +/- 1.8-fold, 3.1 +/- 1.6-fold, and 4.6 +/- 0.8-fold, 3.1 +/- 0.9-fold increases, respectively), were significantly higher than those of the control group. CONCLUSIONS: The administration of Mosapride Citrate for 2 weeks enhanced postprandial plasma active glucagon-like peptide-1 and serum insulin concentration and increased the expression of sweet taste receptors in the upper intestine.
Mosapride citrate improves nonalcoholic steatohepatitis with increased fecal lactic acid bacteria and plasma glucagon-like peptide-1 level in a rodent model.[Pubmed:25428903]
Am J Physiol Gastrointest Liver Physiol. 2015 Jan 15;308(2):G151-8.
Several lines of evidence have suggested a role of gut microbiota in the etiology of nonalcoholic steatohepatitis (NASH). NASH subjects reportedly showed a prolonged orocecal transit time coexistent with small intestinal bacterial overgrowth. We considered the possibility that enhanced gastrointestinal motility would influence gut microbiota and thus investigated the effects of the gastroprokinetic agent Mosapride Citrate (MC) on gut microbiota and the development of NASH using a methionine-choline deficient (MCD) diet-fed rodent model. Mice were divided into three groups, given the normal chow diet (NCD), the MCD diet, or the MCD diet containing 10 mg.kg(-1).day(-1) of MC (MCD plus MC) for 6 wk. NASH development was evaluated based on hepatic histochemical findings, serum parameters and various mRNA and/or protein expression levels. MC treatment suppressed MCD diet-induced NASH development, with reduced serum lipopolysaccharide and increased plasma glucagon-like peptide-1 (GLP-1) concentrations. Calculation of the relative abundance of each strain based on gut microbiota analyses indicated lactic acid bacteria specifically, such as Bifidobacterium and Lactobacillus, in feces to be decreased in the MCD, compared with the NCD group. Interestingly, the reduction in lactic acid bacteria in the MCD diet group was reversed in the MCD plus MC group. In addition, colon inflammation observed in the MCD diet group was reduced in the MCD plus MC group. Therefore, MC showed a protective effect against MCD diet-induced NASH development in our rodent model, with possible involvements of increased fecal lactic acid bacteria, protection against colon inflammation and elevated plasma GLP-1.
Drug-induced Liver Injury Associated with Mosapride Citrate: A Report of Two Cases.[Pubmed:28049998]
Intern Med. 2017;56(1):41-45.
We herein report two cases of drug-induced liver injury (DILI) due to mosapride. Case 1: A 78-year-old man was admitted with elevated transaminase levels. The cessation of mosapride led to the improvement of elevated liver enzyme levels. Case 2: A 54-year-old man was admitted with jaundice. Mosapride was discontinued immediately, and methylprednisolone was administered for acute liver failure. The patient's data showed improvement, and he was discharged on Day 32. In both cases, mosapride gave a positive response to a drug-induced lymphocyte stimulation test (DLST), and the patient's score based on the criteria for DILI was "highly probable".
Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motility in vivo and in vitro.[Pubmed:9399969]
J Pharmacol Exp Ther. 1997 Dec;283(3):1000-8.
Mosapride Citrate is a new gastroprokinetic agent that enhances the upper GI motility by stimulating 5-hydroxytryptamine4 (5-HT4) receptors. The purpose of this study was to compare the effects of mosapride and the existing 5-HT4 receptor agonists on GI motility in conscious dogs and on various 5-HT4 receptor-mediated responses in vitro. In conscious dogs with force transducers implanted, mosapride (0.3-3 mg/kg i.v.) stimulated the antral motility without affecting the colonic motility. However, cisapride, zacopride and BIMU 8 (0. 1-1 mg/kg i.v.) stimulated both antral and colonic motility. The enhanced GI motility induced by mosapride or cisapride was antagonized by pretreatment with GR113808 (1 mg/kg bolus i.v., thereafter 1 mg/kg/hr infusion), a selective 5-HT4 receptor antagonist. In the receptor binding studies, mosapride inhibited [3H]-GR113808 binding to 5-HT4 receptor sites of guinea pig striatum with an IC50 value of 113 nM. In addition, mosapride caused relaxation of the carbachol-precontracted rat esophagus, enhanced the electrically evoked contractions of guinea pig ileum and evoked the contractions of guinea pig distal colon with EC50 values of 208, 73, and 3029 nM, respectively; this indicates that mosapride has a low affinity for colon than for the rest of the GI tract. In contrast, cisapride, zacopride or BIMU 8 had similar potencies in all preparations examined. In conclusion, these studies indicate that mosapride selectively stimulates upper GI motility in vivo and in vitro. These results also suggest heterogeneity of 5-HT4 receptors in the GI tract.
AS-4370, a novel gastrokinetic agent free of dopamine D2 receptor antagonist properties.[Pubmed:2533479]
Arch Int Pharmacodyn Ther. 1989 Jul-Aug;300:51-67.
The gastrokinetic effects of AS-4370, 4-amino-5-chloro-2-ethoxy-N-([4-(4-fluorobenzyl)-2-morpholinyl] methyl) benzamide citrate, were compared with those of metoclopramide in experimental animals. In rats, AS-4370 increased the gastric emptying of a semi-solid meal and of resin pellets at dose ranges of 0.03-30 mg/kg and 1-10 mg/kg p.o., respectively. The minimal effective doses were 3-10 times lower than those of metoclopramide. Gastric emptying, delayed by gastroduodenal surgical intervention, was improved with AS-4370 (0.3-3 mg/kg p.o.). In conscious dogs with strain gauges implanted, AS-4370 (0.5 and 1.0 mg/kg i.v.) enhanced antral and duodenal motility without affecting ileal and colonic motility, indicating a selective enhancing effect on upper gastrointestinal motility. AS-4370 (10(-7) - 3 x 10(-5) M) increased electrically evoked, cholinergically mediated contractions in isolated guinea-pig ileum. AS-4370 (3 mg/kg i.v.) was without effect on gastric acid secretion in anesthetized rats. Unlike metoclopramide, AS-4370 neither depressed the active avoidance response in mice nor the food-reinforced lever pressing response in rats, even at 100 mg/kg p.o. Moreover, AS-4370 (10(-4) M) showed no affinity for D2, alpha 1, alpha 2, 5-HT1 and 5-HT2 binding sites in rat brain synaptic membranes. These results suggest that AS-4370 is a new and potent gastrokinetic agent that lacks dopamine D2 receptor antagonist properties.


