HIV-1 integrase inhibitorUesful for anti-HIV CAS# 544467-07-4 |
- LDE225 Diphosphate
Catalog No.:BCC1693
CAS No.:1218778-77-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- LDE225 (NVP-LDE225,Erismodegib)
Catalog No.:BCC5066
CAS No.:956697-53-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 544467-07-4 | SDF | Download SDF |
| PubChem ID | 503631 | Appearance | Powder |
| Formula | C11H9N3O4 | M.Wt | 247.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
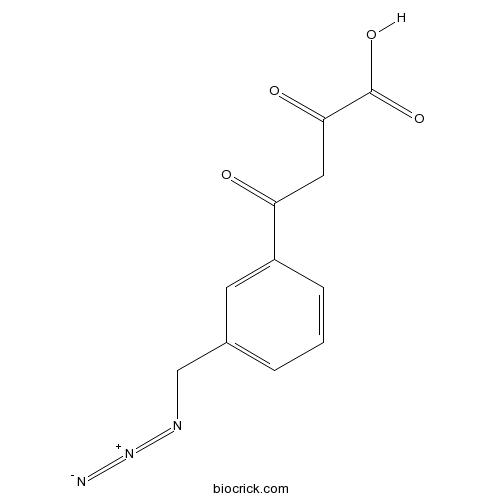
| Synonyms | (Z)-4-(3-(azidomethyl)phenyl)-2-hydroxy-4-oxobut-2-enoic acid | ||
| Solubility | DMSO : ≥ 100 mg/mL (404.51 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[3-(azidomethyl)phenyl]-2,4-dioxobutanoic acid | ||
| SMILES | C1=CC(=CC(=C1)CN=[N+]=[N-])C(=O)CC(=O)C(=O)O | ||
| Standard InChIKey | MXECCJRSXQRLTO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H9N3O4/c12-14-13-6-7-2-1-3-8(4-7)9(15)5-10(16)11(17)18/h1-4H,5-6H2,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | HIV-1 integrase inhibitor is an inhibitor of HIV-1 integrase used for anti-HIV. | |||||
| Targets | HIV-1 integrase | |||||

HIV-1 integrase inhibitor Dilution Calculator

HIV-1 integrase inhibitor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0451 mL | 20.2257 mL | 40.4514 mL | 80.9029 mL | 101.1286 mL |
| 5 mM | 0.809 mL | 4.0451 mL | 8.0903 mL | 16.1806 mL | 20.2257 mL |
| 10 mM | 0.4045 mL | 2.0226 mL | 4.0451 mL | 8.0903 mL | 10.1129 mL |
| 50 mM | 0.0809 mL | 0.4045 mL | 0.809 mL | 1.6181 mL | 2.0226 mL |
| 100 mM | 0.0405 mL | 0.2023 mL | 0.4045 mL | 0.809 mL | 1.0113 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
HIV-1 integrase inhibitor is useful for anti-HIV, with IC50 value of 0.33 µM,[1] which can target HIV-1 integrase and depress the activity in the treatment of HIV infection, AIDS, and other similar diseases characterized by integration of a retroviral genome into a host chromosome.
HIV integrase is a 32 kDa protein produced from the C-terminal portion of the Pol gene product, an enzyme produced by HIV that enables its genetic material to be integrated into the DNA of the infected cell [2]which are not to be confused with phage integrases and a key component in the retroviral pre-integration complex (PIC)[3]. HIV-1 integrase is composed of 3 structurally independent, functional domains: the N-terminal domain (NTD), catalytic core domain (CCD) and the C-terminal domain (CTD).The HIV-1 integration occurs through a multistep process that includes two catalytic reactions: 3’endonucleolytic processing of proviral DNA ends (termed 3’processing) and integration of 3’-processed viral DNA into cellular DNA (referred to as strand transfer)[4].
The human immunodeficiency virus (HIV) is the causative agent for the acquired immunodeficiency syndrome (AIDS)[5], then HIV integrase is an attractive target for new anti-HIV drugs. The drug design of HIV-1 integrase inhibitor include integrase strand transfer inhibitors (INSTIs),inhibition of the LEDGF/p75- integrase interaction and integrase binding inhibitors, but strand transfer inhibition is the most intuitively obvious and readily pursued to date.Mg2+ and Mn2+ are critical cofactors in the integration phase, so removing these cofactors (e.g. through chelation) causes functional impairment of integrase[6].Competitive inhibitors compete directly with viral DNA for binding to integrase in order to inhibit 3‘-end processing.[7] In doing this the inhibitors completely block the active site from binding to target DNA.INSTIs bind tightly and specifically to the IN that is associated with the ends of the DNA by chelating the divalent metal ions (Mg2+) which is coordinated by the catalytic triad, such as the DDE motif which is located in the CCD and is the active site of the enzyme[8].
Development of a successful INSTI treatment was accomplished when raltegravir was discovered by Merck Sharp & Dohme Limited.[9] S/GSK1349572 is an integrase inhibitor discovered by ViiV/Shinongi which was entering phase three in clinical trials in 2011. This new drug is promising and seems to be well tolerated and so far shows better results than both raltegravir and elvitegravir.[10]
References:
1.Loizidou EZ et al. Analysis of binding parameters of HIV-1 integrase inhibitors: correlates of drug inhibition and resistance. Bioorg Med Chem. 2009, 17(13):4806-18.
2.Cocohoba, J; Dong, BJ. "Raltegravir: the first HIV integrase inhibitor". Clinical therapeutics.2008, 30(10): 1747–65.
3.Mouscadet, JF; Delelis, O; Marcelin, AG; Tchertanov, L. "Resistance to HIV-1 integrase inhibitors: A structural perspective". Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy.2010, 13(4-5):139–50.
4.Fan, X; Zhang, FH.et al."Design of HIV-1 integrase inhibitors targeting the catalytic domain as well as its interaction with LEDGF/p75: a scaffold hopping approach using salicylate and catechol groups". Bioorganic & Medicinal Chemistry.2011,19 (16): 4935–52.
5.Pommier, Yves.et al. "Integrase inhibitors to treat HIV/Aids". Nature Reviews Drug Discovery.2005, 4 (3): 236–248.
6.Pendri, A.et al. "New first and second generation inhibitors of human immunodeficiency virus-1 integrase". Expert opinion on therapeutic patents. 2011,21 (8): 1173–89.
7.Chen, X; Tsiang, M, Yu, F, Hung, M, Jones, GS, Zeynalzadegan, A, Qi, X, Jin, H, Kim, CU, Swaminathan, S, Chen, JM. "Modeling, analysis, and validation of a novel HIV integrase structure provide insights into the binding modes of potent integrase inhibitors". Journal of Molecular Biology. 2008, 380 (3): 504–19.
8.Mouscadet, JF. et al."Resistance to HIV-1 integrase inhibitors: A structural perspective". Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy.2010,13(4-5):139–50.
9.McColl, DJ; Chen, X. "Strand transfer inhibitors of HIV-1 integrase: bringing IN a new era of antiretroviral therapy". Antiviral Research,. 2010,85 (1): 101–18.
10.Barnhart, Matthew,James Shelton."A better state of ART improving antiretroviral regimens to increase global access to HIV treatment". Journal of AIDS and HIV Research. 2011, 3 (4): 71–78.
- Capadenoson
Catalog No.:BCC1450
CAS No.:544417-40-5
- Norcantharidin
Catalog No.:BCN1281
CAS No.:5442-12-6
- Myristic acid
Catalog No.:BCN8390
CAS No.:544-63-8
- Palmitoylethanolamide
Catalog No.:BCC6828
CAS No.:544-31-0
- Lirinidine
Catalog No.:BCN8274
CAS No.:54383-28-7
- N,N-Bis(2-hydroxyethyl)-p-phenylenediamine sulphate
Catalog No.:BCN8366
CAS No.:54381-16-7
- 7-Hydroxy-5,8-dimethoxyflavanone
Catalog No.:BCN5722
CAS No.:54377-24-1
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- 4'-Methoxyacetoacetanilide
Catalog No.:BCC8712
CAS No.:5437-98-9
- Decarine
Catalog No.:BCN5721
CAS No.:54354-62-0
- Eriodictyol 7,3'-dimethyl ether
Catalog No.:BCN8105
CAS No.:54352-60-2
- 4beta-Hydroxywithanolide E
Catalog No.:BCN7572
CAS No.:54334-04-2
- c-di-AMP
Catalog No.:BCC8054
CAS No.:54447-84-6
- MRS 1845
Catalog No.:BCC7198
CAS No.:544478-19-5
- UBP 282
Catalog No.:BCC7171
CAS No.:544697-47-4
- JNJ 10181457 dihydrochloride
Catalog No.:BCC7842
CAS No.:544707-20-2
- 3alpha-dihydrocadambine
Catalog No.:BCN8151
CAS No.:54483-84-0
- Jolkinolide E
Catalog No.:BCN3772
CAS No.:54494-34-7
- 5-Glutinen-3-ol
Catalog No.:BCN5723
CAS No.:545-24-4
- Uvaol
Catalog No.:BCN5724
CAS No.:545-46-0
- Lupeol
Catalog No.:BCN5725
CAS No.:545-47-1
- Erythrodiol
Catalog No.:BCN5726
CAS No.:545-48-2
- 5-Aminolevulinic acid HCl
Catalog No.:BCC4883
CAS No.:5451-09-2
- H-Leu-CMK.HCl
Catalog No.:BCC2971
CAS No.:54518-92-2
Asymmetric Synthesis of a Potent HIV-1 Integrase Inhibitor.[Pubmed:27471910]
J Org Chem. 2016 Nov 4;81(21):10256-10265.
The development of a practical asymmetric total synthesis of the potent HIV-1 integrase inhibitor 5 is described. Key transformations include construction of the naphthridine core in a highly efficient manner followed by cyclization of the 8-membered ring. Control of the atropisomers of intermediates and final compound 5 is also described.
Switching regimens in virologically suppressed HIV-1-infected patients: evidence base and rationale for integrase strand transfer inhibitor (INSTI)-containing regimens.[Pubmed:27714978]
HIV Med. 2016 Oct;17 Suppl 5:3-16.
In an era when most individuals with treated HIV infection can expect to live into old age, clinicians should proactively review their patients' current and future treatment needs and challenges. Clinical guidelines acknowledge that, in the setting of virological suppression, treatment switch may yield benefits in terms of tolerability, regimen simplification, adherence, convenience and long-term health considerations, particularly in the context of ageing. In this paper, we review evidence from six key clinical studies on switching virologically suppressed patients to regimens based on integrase strand transfer inhibitors (INSTIs), the antiretroviral class increasingly preferred as initial therapy in clinical guidelines. We review these studies and focus on the virological efficacy, safety, and tolerability of switching to INSTI-based regimens in suppressed HIV-positive individuals. We review the early switch studies SWITCHMRK and SPIRAL [assessing a switch from a ritonavir-boosted protease inhibitor (PI/r) to raltegravir (RAL)-containing regimens], together with data from STRATEGY-PI [assessing a switch to elvitegravir (EVG)-containing regimens; EVG/cobicistat (COBI)/emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) vs. remaining on a PI/r-containing regimen], STRATEGY-NNRTI [assessing a switch to EVG/COBI/FTC/TDF vs. continuation of a nonnucleoside reverse transcriptase inhibitor (NNRTI) and two nucleoside reverse transcriptase inhibitors (NRTIs)], STRIIVING [assessing a switch to a dolutegravir (DTG)-containing regimen (abacavir (ABC)/lamivudine (3TC)/DTG) vs. staying on the background regimen], and GS study 109 [assessing a switch to EVG/COBI/FTC/tenofovir alafenamide fumarate (TAF) vs. continuation of FTC/TDF-based regimens]. Switching to INSTI-containing regimens has been shown to support good virological efficacy, with evidence from two studies demonstrating superior virological efficacy for a switch to EVG-containing regimens. In addition, switching to INSTI regimens was associated with improved tolerability and greater reported patient satisfaction and outcomes in some studies. INSTI-based regimens offer an important contemporary switch option that may be tailored to meet and optimize the needs of many patients.
Peptide fibrils as monomer storage of the covalent HIV-1 integrase inhibitor.[Pubmed:28070909]
J Pept Sci. 2017 Feb;23(2):117-121.
We have recently reported the covalent inhibition of HIV-1 integrase by an N-terminal succinimide-modified lens epithelium-derived growth factor (361-370) peptide. We also showed that this peptide is proteolytically stable. Here, we show that this inhibitor is stored as fibrils that serve as a stock for the inhibitory monomers. The fibrils increase the local concentration of the peptide at the target protein. When the monomers bind integrase, the equilibrium between the fibrils and their monomers shifts towards the formation of peptide monomers. The combination of fibril formation and subsequent proteolytic stability of the peptide may bring to new strategy for developing therapeutic agents. Copyright (c) 2017 European Peptide Society and John Wiley & Sons, Ltd.
Lack of impact of pre-existing T97A HIV-1 integrase mutation on integrase strand transfer inhibitor resistance and treatment outcome.[Pubmed:28212411]
PLoS One. 2017 Feb 17;12(2):e0172206.
T97A is an HIV-1 integrase polymorphism associated with integrase strand transfer inhibitor (INSTI) resistance. Using pooled data from 16 clinical studies, we investigated the prevalence of T97A (pre-existing and emergent) and its impact on INSTI susceptibility and treatment response in INSTI-naive patients who enrolled on elvitegravir (EVG)- or raltegravir (RAL)-based regimens. Prior to INSTI-based therapy, primary INSTI resistance-associated mutations (RAMs) were absent and T97A pre-existed infrequently (1.4%; 47 of 3367 integrase sequences); most often among non-B (5.3%) than B (0.9%) HIV-1 subtypes. During INSTI-based therapy, few patients experienced virologic failure with emergent INSTI RAMs (3%; 122 of 3881 patients), among whom T97A emerged infrequently in the presence (n = 6) or absence (n = 8) of primary INSTI RAMs. A comparison between pre-existing and emergent T97A patient populations (i.e., in the absence of primary INSTI RAMs) showed no significant differences in EVG or RAL susceptibility in vitro. Furthermore, among all T97A-containing viruses tested, only 38-44% exhibited reduced susceptibility to EVG and/or RAL (all of low magnitude; <11-fold), while all maintained susceptibility to dolutegravir. Of the patients with pre-existing T97A, 17 had available clinical follow-up: 16 achieved virologic suppression and 1 maintained T97A and INSTI sensitivity without further resistance development. Overall, T97A is an infrequent integrase polymorphism that is enriched among non-B HIV-1 subtypes and can confer low-level reduced susceptibility to EVG and/or RAL. However, detection of T97A does not affect response to INSTI-based therapy with EVG or RAL. These results suggest a very low risk of initiating INSTI-based therapy in patients with pre-existing T97A.


