c-di-AMPCAS# 54447-84-6 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

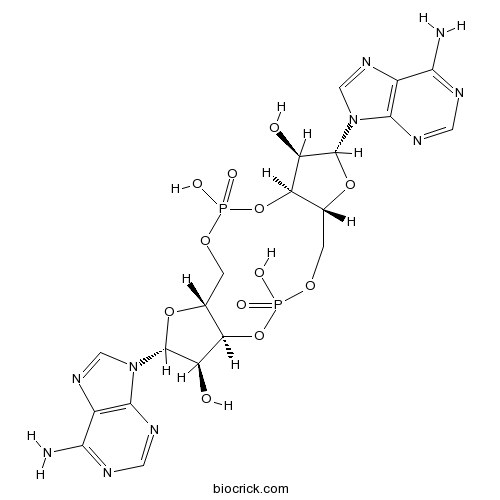
| Cas No. | 54447-84-6 | SDF | Download SDF |
| PubChem ID | 11158091 | Appearance | Powder |
| Formula | C20H24N10O12P2 | M.Wt | 658.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Cyclic diadenylate; Cyclic-di-AMP | ||
| Solubility | H2O : 5.5 mg/mL (8.35 mM; Need ultrasonic) | ||
| Chemical Name | (1S,6R,8R,9R,10S,15R,17R,18R)-8,17-bis(6-aminopurin-9-yl)-3,12-dihydroxy-3,12-dioxo-2,4,7,11,13,16-hexaoxa-3λ5,12λ5-diphosphatricyclo[13.3.0.06,10]octadecane-9,18-diol | ||
| SMILES | C1C2C(C(C(O2)N3C=NC4=C3N=CN=C4N)O)OP(=O)(OCC5C(C(C(O5)N6C=NC7=C6N=CN=C7N)O)OP(=O)(O1)O)O | ||
| Standard InChIKey | PDXMFTWFFKBFIN-XPWFQUROSA-N | ||
| Standard InChI | InChI=1S/C20H24N10O12P2/c21-15-9-17(25-3-23-15)29(5-27-9)19-11(31)13-7(39-19)1-37-43(33,34)42-14-8(2-38-44(35,36)41-13)40-20(12(14)32)30-6-28-10-16(22)24-4-26-18(10)30/h3-8,11-14,19-20,31-32H,1-2H2,(H,33,34)(H,35,36)(H2,21,23,25)(H2,22,24,26)/t7-,8-,11-,12-,13-,14-,19-,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclic-di-AMP (c-di-AMP) is a second messenger molecule produced in bacteria but not in mammals; can induce a strong immune response in vitro and in vivo. References: | |||||

c-di-AMP Dilution Calculator

c-di-AMP Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5188 mL | 7.5941 mL | 15.1881 mL | 30.3762 mL | 37.9703 mL |
| 5 mM | 0.3038 mL | 1.5188 mL | 3.0376 mL | 6.0752 mL | 7.5941 mL |
| 10 mM | 0.1519 mL | 0.7594 mL | 1.5188 mL | 3.0376 mL | 3.797 mL |
| 50 mM | 0.0304 mL | 0.1519 mL | 0.3038 mL | 0.6075 mL | 0.7594 mL |
| 100 mM | 0.0152 mL | 0.0759 mL | 0.1519 mL | 0.3038 mL | 0.3797 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cyclic-di-AMP (c-di-AMP) is a second messenger molecule produced in bacteria but not in mammals; can induce a strong immune response in vitro and in vivo.
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- Capadenoson
Catalog No.:BCC1450
CAS No.:544417-40-5
- Norcantharidin
Catalog No.:BCN1281
CAS No.:5442-12-6
- Myristic acid
Catalog No.:BCN8390
CAS No.:544-63-8
- Palmitoylethanolamide
Catalog No.:BCC6828
CAS No.:544-31-0
- Lirinidine
Catalog No.:BCN8274
CAS No.:54383-28-7
- N,N-Bis(2-hydroxyethyl)-p-phenylenediamine sulphate
Catalog No.:BCN8366
CAS No.:54381-16-7
- 7-Hydroxy-5,8-dimethoxyflavanone
Catalog No.:BCN5722
CAS No.:54377-24-1
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- 4'-Methoxyacetoacetanilide
Catalog No.:BCC8712
CAS No.:5437-98-9
- Decarine
Catalog No.:BCN5721
CAS No.:54354-62-0
- Eriodictyol 7,3'-dimethyl ether
Catalog No.:BCN8105
CAS No.:54352-60-2
- MRS 1845
Catalog No.:BCC7198
CAS No.:544478-19-5
- UBP 282
Catalog No.:BCC7171
CAS No.:544697-47-4
- JNJ 10181457 dihydrochloride
Catalog No.:BCC7842
CAS No.:544707-20-2
- 3alpha-dihydrocadambine
Catalog No.:BCN8151
CAS No.:54483-84-0
- Jolkinolide E
Catalog No.:BCN3772
CAS No.:54494-34-7
- 5-Glutinen-3-ol
Catalog No.:BCN5723
CAS No.:545-24-4
- Uvaol
Catalog No.:BCN5724
CAS No.:545-46-0
- Lupeol
Catalog No.:BCN5725
CAS No.:545-47-1
- Erythrodiol
Catalog No.:BCN5726
CAS No.:545-48-2
- 5-Aminolevulinic acid HCl
Catalog No.:BCC4883
CAS No.:5451-09-2
- H-Leu-CMK.HCl
Catalog No.:BCC2971
CAS No.:54518-92-2
- Methyl protodioscin
Catalog No.:BCN6342
CAS No.:54522-52-0
A New Second Messenger: Bacterial c-di-AMP.[Pubmed:27910745]
Crit Rev Eukaryot Gene Expr. 2016;26(4):309-316.
Nucleotide-based second messengers transduce signals originating from both outside and inside the cell to adaptive responses accordingly. c-di-AMP is a newly established second messenger employed by many organisms. We summarize recent advances in bacterial c-di-AMP-mediated signaling, especially the interaction between c-di-AMP signaling and the host.
Immunization with Tc52 or its amino terminal domain adjuvanted with c-di-AMP induces Th17+Th1 specific immune responses and confers protection against Trypanosoma cruzi.[Pubmed:28234897]
PLoS Negl Trop Dis. 2017 Feb 24;11(2):e0005300.
The development of new adjuvants enables fine modulation of the elicited immune responses. Ideally, the use of one or more adjuvants should result in the induction of a protective immune response against the specific pathogen. We have evaluated the immune response and protection against Trypanosoma cruzi infection in mice vaccinated with recombinant Tc52 or its N- and C-terminal domains (NTc52 and CTc52) adjuvanted either with the STING (Stimulator of Interferon Genes) agonist cyclic di-AMP (c-di-AMP), a pegylated derivative of alpha-galactosylceramide (alphaGC-PEG), or oligodeoxynucleotides containing unmethylated CpG motifs (ODN-CpG). All groups immunized with the recombinant proteins plus adjuvant: Tc52+c-di-AMP, NTc52+c-di-AMP, CTc52+c-di-AMP, NTc52+c-di-AMP+alphaGC-PEG, NTc52+CpG, developed significantly higher anti-Tc52 IgG titers than controls. Groups immunized with c-di-AMP and Tc52, NTc52 or CTc52 showed the highest Tc52-specific IgA titers in nasal lavages. All groups immunized with the recombinant proteins plus adjuvant developed a strong specific cellular immune response in splenocytes and lymph node cells with significant differences for groups immunized with c-di-AMP and Tc52, NTc52 or CTc52. These groups also showed high levels of Tc52-specific IL-17 and IFN-gamma producing cells, while NTc52+CpG group only showed significant difference with control in IFN-gamma producing cells. Groups immunized with c-di-AMP and Tc52, NTc52 or CTc52 developed predominantly a Th17 and Th1immune response, whereas for NTc52+CpG it was a dominant Th1 response. It was previously described that alphaGC-PEG inhibits Th17 differentiation by activating NKT cells. Thus, in this work we have also included a group immunized with both adjuvants (NTc52+c-di-AMP+alphaGC-PEG) with the aim to modulate the Th17 response induced by c-di-AMP. This group showed a significant reduction in the number of Tc52-specific IL-17 producing splenocytes, as compared to the group NTc52+c-di-AMP, which has in turn correlated with a reduction in protection against infection. These results suggest that the Th17 immune response developed after immunizing with NTc52+c-di-AMP could have a protective role against T. cruzi infection. Groups NTc52+c-di-AMP, Tc52+c-di-AMP and NTc52PB, were the ones that showed better protection against infection with lower parasitemia and weight loss, and higher survival.
New Insights into the Cyclic Di-adenosine Monophosphate (c-di-AMP) Degradation Pathway and the Requirement of the Cyclic Dinucleotide for Acid Stress Resistance in Staphylococcus aureus.[Pubmed:27834680]
J Biol Chem. 2016 Dec 30;291(53):26970-26986.
Nucleotide signaling networks are key to facilitate alterations in gene expression, protein function, and enzyme activity in response to diverse stimuli. Cyclic di-adenosine monophosphate (c-di-AMP) is an important secondary messenger molecule produced by the human pathogen Staphylococcus aureus and is involved in regulating a number of physiological processes including potassium transport. S. aureus must ensure tight control over its cellular levels as both high levels of the dinucleotide and its absence result in a number of detrimental phenotypes. Here we show that in addition to the membrane-bound Asp-His-His and Asp-His-His-associated (DHH/DHHA1) domain-containing phosphodiesterase (PDE) GdpP, S. aureus produces a second cytoplasmic DHH/DHHA1 PDE Pde2. Although capable of hydrolyzing c-di-AMP, Pde2 preferentially converts linear 5'-phosphadenylyl-adenosine (pApA) to AMP. Using a pde2 mutant strain, pApA was detected for the first time in S. aureus, leading us to speculate that this dinucleotide may have a regulatory role under certain conditions. Moreover, pApA is involved in a feedback inhibition loop that limits GdpP-dependent c-di-AMP hydrolysis. Another protein linked to the regulation of c-di-AMP levels in bacteria is the predicted regulator protein YbbR. Here, it is shown that a ybbR mutant S. aureus strain has increased acid sensitivity that can be bypassed by the acquisition of mutations in a number of genes, including the gene coding for the diadenylate cyclase DacA. We further show that c-di-AMP levels are slightly elevated in the ybbR suppressor strains tested as compared with the wild-type strain. With this, we not only identified a new role for YbbR in acid stress resistance in S. aureus but also provide further insight into how c-di-AMP levels impact acid tolerance in this organism.
c-di-AMP modulates Listeria monocytogenes central metabolism to regulate growth, antibiotic resistance and osmoregulation.[Pubmed:28097715]
Mol Microbiol. 2017 Apr;104(2):212-233.
Cyclic diadenosine monophosphate (c-di-AMP) is a conserved nucleotide second messenger critical for bacterial growth and resistance to cell wall-active antibiotics. In Listeria monocytogenes, the sole diadenylate cyclase, DacA, is essential in rich, but not synthetic media and DeltadacA mutants are highly sensitive to the beta-lactam antibiotic cefuroxime. In this study, loss of function mutations in the oligopeptide importer (oppABCDF) and glycine betaine importer (gbuABC) allowed DeltadacA mutants to grow in rich medium. Since oligopeptides were sufficient to inhibit growth of the DeltadacA mutant we hypothesized that oligopeptides act as osmolytes, similar to glycine betaine, to disrupt intracellular osmotic pressure. Supplementation with salt stabilized the DeltadacA mutant in rich medium and restored cefuroxime resistance. Additional suppressor mutations in the acetyl-CoA binding site of pyruvate carboxylase (PycA) rescued cefuroxime resistance and resulted in a 100-fold increase in virulence of the DeltadacA mutant. PycA is inhibited by c-di-AMP and these mutations prompted us to examine the role of TCA cycle enzymes. Inactivation of citrate synthase, but not down-stream enzymes suppressed DeltadacA phenotypes. These data suggested that c-di-AMP modulates central metabolism at the pyruvate node to moderate citrate production and indeed, the DeltadacA mutant accumulated six times the concentration of citrate present in wild-type bacteria.


