ErythrodiolCAS# 545-48-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 545-48-2 | SDF | Download SDF |
| PubChem ID | 101761 | Appearance | White powder |
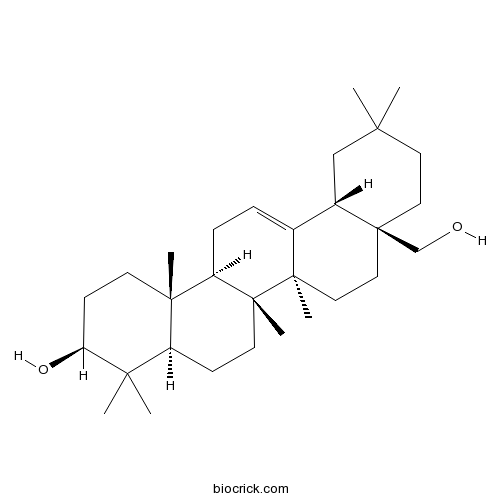
| Formula | C30H50O2 | M.Wt | 442.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform; insoluble in water | ||
| Chemical Name | (3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-(hydroxymethyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-ol | ||
| SMILES | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1)C)CO)C | ||
| Standard InChIKey | PSZDOEIIIJFCFE-OSQDELBUSA-N | ||
| Standard InChI | InChI=1S/C30H50O2/c1-25(2)14-16-30(19-31)17-15-28(6)20(21(30)18-25)8-9-23-27(5)12-11-24(32)26(3,4)22(27)10-13-29(23,28)7/h8,21-24,31-32H,9-19H2,1-7H3/t21-,22-,23+,24-,27-,28+,29+,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Erythrodiol is the precursor of pentacyclic triterpenic acids, it exerts antiproliferative and proapoptotic activity in colon adenocarcinoma cells. Erythrodiol may have interesting therapeutic potential as new vasodilator drugs, thus protecting the cardiovascular system. |
| Targets | Caspase | NOS |
| In vitro | Erythrodiol, a natural triterpenoid from olives, has antiproliferative and apoptotic activity in HT-29 human adenocarcinoma cells.[Pubmed: 18384095]Mol Nutr Food Res. 2008 May;52(5):595-9.Erythrodiol is the precursor of pentacyclic triterpenic acids present in Olea Europaea. Although olive oil and some of its constituents are reported to have anticarcinogenic activities, Erythrodiol has not been assessed in its cell biological functions in detail.
We therefore determined its effects on cell growth and apoptosis in human colorectal carcinoma HT-29 cells.
Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in 'orujo' olive oil, on rat aorta.[Pubmed: 15522132]Br J Nutr. 2004 Oct;92(4):635-42.'Orujo' olive oil is obtained by chemical processes from the waste resulting from the mechanical extraction of virgin olive oil. The aim of the present study was to evaluate a new pharmacological property of two natural triterpenoids contained in olive oil, as vasodilatory agents, and to determine their mechanism of action. The two compounds studied were oleanolic acid and Erythrodiol.
|
| Structure Identification | J Membr Biol. 2015 Dec;248(6):1079-87.Effect of Erythrodiol, A Natural Pentacyclic Triterpene from Olive Oil, on the Lipid Membrane Properties.[Pubmed: 26141679]The effect of Erythrodiol, a natural pentacyclic triterpene to which humans are exposed through nutrients, on the lipid membranes is studied using liposomes as a membrane model.
|

Erythrodiol Dilution Calculator

Erythrodiol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2589 mL | 11.2943 mL | 22.5887 mL | 45.1773 mL | 56.4717 mL |
| 5 mM | 0.4518 mL | 2.2589 mL | 4.5177 mL | 9.0355 mL | 11.2943 mL |
| 10 mM | 0.2259 mL | 1.1294 mL | 2.2589 mL | 4.5177 mL | 5.6472 mL |
| 50 mM | 0.0452 mL | 0.2259 mL | 0.4518 mL | 0.9035 mL | 1.1294 mL |
| 100 mM | 0.0226 mL | 0.1129 mL | 0.2259 mL | 0.4518 mL | 0.5647 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lupeol
Catalog No.:BCN5725
CAS No.:545-47-1
- Uvaol
Catalog No.:BCN5724
CAS No.:545-46-0
- 5-Glutinen-3-ol
Catalog No.:BCN5723
CAS No.:545-24-4
- Jolkinolide E
Catalog No.:BCN3772
CAS No.:54494-34-7
- 3alpha-dihydrocadambine
Catalog No.:BCN8151
CAS No.:54483-84-0
- JNJ 10181457 dihydrochloride
Catalog No.:BCC7842
CAS No.:544707-20-2
- UBP 282
Catalog No.:BCC7171
CAS No.:544697-47-4
- MRS 1845
Catalog No.:BCC7198
CAS No.:544478-19-5
- c-di-AMP
Catalog No.:BCC8054
CAS No.:54447-84-6
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- Capadenoson
Catalog No.:BCC1450
CAS No.:544417-40-5
- Norcantharidin
Catalog No.:BCN1281
CAS No.:5442-12-6
- 5-Aminolevulinic acid HCl
Catalog No.:BCC4883
CAS No.:5451-09-2
- H-Leu-CMK.HCl
Catalog No.:BCC2971
CAS No.:54518-92-2
- Methyl protodioscin
Catalog No.:BCN6342
CAS No.:54522-52-0
- Methyl protogracillin
Catalog No.:BCN8177
CAS No.:54522-53-1
- Nicardipine HCl
Catalog No.:BCC4685
CAS No.:54527-84-3
- QNZ (EVP4593)
Catalog No.:BCC2249
CAS No.:545380-34-5
- AMG 9810
Catalog No.:BCC7329
CAS No.:545395-94-6
- Quercetin-3-O-glucose-6'-acetate
Catalog No.:BCN6545
CAS No.:54542-51-7
- Doxercalciferol
Catalog No.:BCC4902
CAS No.:54573-75-0
- Conessine
Catalog No.:BCC7352
CAS No.:546-06-5
- Alantolactone
Catalog No.:BCN1033
CAS No.:546-43-0
- α-Thujone
Catalog No.:BCC8271
CAS No.:546-80-5
Erythrodiol, a natural triterpenoid from olives, has antiproliferative and apoptotic activity in HT-29 human adenocarcinoma cells.[Pubmed:18384095]
Mol Nutr Food Res. 2008 May;52(5):595-9.
Erythrodiol is the precursor of pentacyclic triterpenic acids present in Olea Europaea. Although olive oil and some of its constituents are reported to have anticarcinogenic activities, Erythrodiol has not been assessed in its cell biological functions in detail. We therefore determined its effects on cell growth and apoptosis in human colorectal carcinoma HT-29 cells. Proliferation, cytotoxicity, and apoptosis were measured by fluorescence-based techniques. Erythrodiol inhibited cell growth with an EC50 value of 48.8 +/- 3.7 microM without any cytotoxic effects in a concentration range up to 100 microM. However, exposure of cells for 24 h to 50, 100, and 150 microM Erythrodiol increased caspase-3-like activity by 3.2-, 4.8-, and 5.2-fold over that in control cells. We here demonstrate for the first time that, in colon adenocarcinoma cells, Erythrodiol exerts antiproliferative and proapoptotic activity.
Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in 'orujo' olive oil, on rat aorta.[Pubmed:15522132]
Br J Nutr. 2004 Oct;92(4):635-42.
'Orujo' olive oil is obtained by chemical processes from the waste resulting from the mechanical extraction of virgin olive oil. The aim of the present study was to evaluate a new pharmacological property of two natural triterpenoids contained in olive oil, as vasodilatory agents, and to determine their mechanism of action. The two compounds studied were oleanolic acid and Erythrodiol. The vasorelaxant effect induced by these pentacyclic triterpenoids was studied in isolated thoracic rat aorta. Oleanolic acid and Erythrodiol, accumulatively added, showed vasorelaxant activities in aortic rings with endothelium pre-contracted by 10(-6) m-phenylephrine (maximum percentage of relaxation 86.38 (sem 2.89) and 73.53 (sem 6.01), respectively). They had almost no relaxant effect on depolarised or endothelium-denuded aortic segments. The relaxation was significantly attenuated by pre-treatment with the NO synthase inhibitor N(omega)-nitro-L-arginine-methylester (L-NAME; 3x10(-4) m). To characterise the involvement of endothelial factors, in addition to NO, arteries with endothelium were exposed to 10(-5) m-indomethacin (INDO), a cyclo-oxygenase inhibitor, or INDO plus L-NAME. INDO did not have any significant effect on the relaxant response of both compounds. The combination of L-NAME plus INDO only abolished the oleanolic acid-induced relaxation. The present results suggest that the mechanism of relaxation seems to be mainly mediated by the endothelial production of NO; however, other mechanisms cannot be excluded. It can be concluded that oleanolic acid and Erythrodiol may have interesting therapeutic potential as new vasodilator drugs, thus protecting the cardiovascular system. Therefore, the intake of 'orujo' olive oil, as a source of these compounds, might be beneficial in this regard.
Effect of Erythrodiol, A Natural Pentacyclic Triterpene from Olive Oil, on the Lipid Membrane Properties.[Pubmed:26141679]
J Membr Biol. 2015 Dec;248(6):1079-87.
The effect of Erythrodiol, a natural pentacyclic triterpene to which humans are exposed through nutrients, on the lipid membranes is studied using liposomes as a membrane model. Empty and Erythrodiol-loaded liposomes were prepared by the reverse phase evaporation method followed by the extrusion and by the thin film hydration method. Liposomes were characterized in terms of size and zeta potential and were imaged by transmission electron microscopy (TEM) and atomic force microscopy (AFM). The effect of Erythrodiol on thermotropic behavior of DPPC bilayers is also examined by differential scanning calorimetry (DSC). The DSC thermograms suggested that Erythrodiol interacted with the polar head groups of phospholipids and may produce a disruption of the ordering of the alkyl chains. The diffraction light scattering analysis showed that Erythrodiol-loaded liposomes presented a decrease in the vesicle size when compared to blank liposomes. Images obtained by TEM confirmed the formation of unilamellar and spherical liposomes. AFM images showed spherical vesicles and single lipid bilayers. The latter were more abundant in the preparations containing Erythrodiol than in the blank ones. Moreover, Erythrodiol-loaded liposomes tended to rupture into single lipid bilayers during scanning. The study may provide a better understanding of pentacyclic triterpenes-membrane interaction.


