Bendamustine HClCytostatic agent for non-Hodgkin lymphomas CAS# 3543-75-7 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3543-75-7 | SDF | Download SDF |
| PubChem ID | 77082 | Appearance | Powder |
| Formula | C16H22Cl3N3O2 | M.Wt | 394.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SDX-105; EP-3101 | ||
| Solubility | DMSO : 100 mg/mL (253.34 mM; Need ultrasonic) | ||
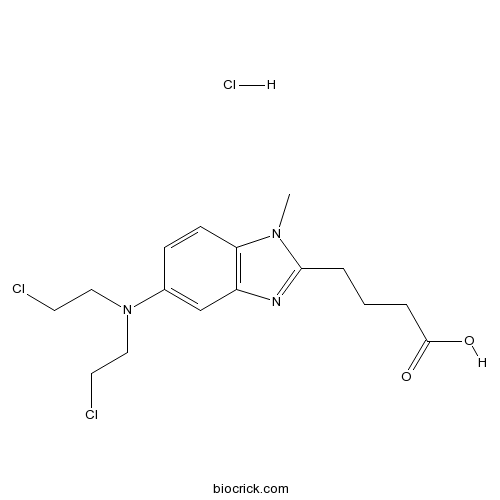
| Chemical Name | 4-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid;hydrochloride | ||
| SMILES | CN1C2=C(C=C(C=C2)N(CCCl)CCCl)N=C1CCCC(=O)O.Cl | ||
| Standard InChIKey | ZHSKUOZOLHMKEA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H21Cl2N3O2.ClH/c1-20-14-6-5-12(21(9-7-17)10-8-18)11-13(14)19-15(20)3-2-4-16(22)23;/h5-6,11H,2-4,7-10H2,1H3,(H,22,23);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cytostatic agent that displays activity in non-Hodgkin's lymphomas. Exhibits bifunctionality; combines DNA alkylating properties with those of purine analogs. |

Bendamustine HCl Dilution Calculator

Bendamustine HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5334 mL | 12.6672 mL | 25.3344 mL | 50.6688 mL | 63.336 mL |
| 5 mM | 0.5067 mL | 2.5334 mL | 5.0669 mL | 10.1338 mL | 12.6672 mL |
| 10 mM | 0.2533 mL | 1.2667 mL | 2.5334 mL | 5.0669 mL | 6.3336 mL |
| 50 mM | 0.0507 mL | 0.2533 mL | 0.5067 mL | 1.0134 mL | 1.2667 mL |
| 100 mM | 0.0253 mL | 0.1267 mL | 0.2533 mL | 0.5067 mL | 0.6334 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bendamustine HCl is an alkylating agent associated with DNA damage with IC50 of 50 μM [1].
It has been reported that bendamustine is activated under DNA damage stress and apoptosis. Bendamustine inhibits mitotic checkpoints and induces mitotic catastrophe by inhibiting several mitosis-related genes such as Polo-like kinase 1, Aurora kinase A, and Cyclin B1 [1]. In myeloma cell lines, bendamustine induced apoptosis by cleavage of caspase 3, and resulted in G2 cell cycle arrest [2]. In chronic lymphocytic and mantle cell lymphoma cell lines, bendamustine HCL has been shown to activate both the mitochondrial cell death pathway and caspase-dependent apoptosis. Some assays showed that bendamustine exhibited anti-proliferation effects on dexamethasone-sensitive (MM1.S) and -resistant (MM1.R) multiple myeloma cells in a dose-dependent manner, with IC50s of 119.8 μM (MM1.S) and 138 μM (MM1.R), respectively. The apoptosis by activation of caspase-3 and caspase-8 was induced in both MM1.S and MM1.R cell lines [4].
References:
[1]. Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, Elliott G, Niemeyer CC. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008 Jan 1;14(1):309-17.
[2]. Gaul L, Mandl-Weber S, Baumann P, Emmerich B, Schmidmaier R. Bendamustine induces G2 cell cycle arrest and apoptosis in myeloma cells: the role of ATM-Chk2-Cdc25A and ATM-p53-p21-pathways. J Cancer Res Clin Oncol. 2008 Feb;134(2):245-53.
[3]. Roué G, López-Guerra M, Milpied P, Pérez-Galán P, Villamor N, Montserrat E, Campo E, Colomer D. Bendamustine is effective in p53-deficient B-cell neoplasms and requires oxidative stress and caspase-independent signaling. Clin Cancer Res. 2008 Nov 1;14(21):6907-15.
[4]. Cai B, Wang S, Huang J, Lee CK, Gao C, Liu B. Cladribine and bendamustine exhibit inhibitory activity in dexamethasone-sensitive and -resistant multiple myeloma cells. Am J Transl Res. 2013;5(1):36-46.
- 5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8724
CAS No.:3543-74-6
- 1-Methyl-5-amino-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8469
CAS No.:3543-73-5
- 1-Methyl-5-nitro-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8470
CAS No.:3543-72-4
- Norathyriol
Catalog No.:BCN5294
CAS No.:3542-72-1
- J 113863
Catalog No.:BCC7422
CAS No.:353791-85-2
- Honokiol
Catalog No.:BCN1001
CAS No.:35354-74-6
- HLM006474
Catalog No.:BCC5403
CAS No.:353519-63-8
- 9-Hydroxycalabaxanthone
Catalog No.:BCN5293
CAS No.:35349-68-9
- TQS
Catalog No.:BCC7896
CAS No.:353483-92-8
- Monocrotaline N-oxide
Catalog No.:BCN2097
CAS No.:35337-98-5
- S26948
Catalog No.:BCC7751
CAS No.:353280-43-0
- NS 6180
Catalog No.:BCC6307
CAS No.:353262-04-1
- INO-1001
Catalog No.:BCC2212
CAS No.:3544-24-9
- Caraphenol A
Catalog No.:BCN5295
CAS No.:354553-35-8
- Hirsuteine
Catalog No.:BCN2756
CAS No.:35467-43-7
- Corianin
Catalog No.:BCN5296
CAS No.:35481-77-7
- SC-514
Catalog No.:BCC4554
CAS No.:354812-17-2
- Balicatib
Catalog No.:BCC5139
CAS No.:354813-19-7
- 3-beta-O-(trans-p-Coumaroyl)maslinic acid
Catalog No.:BCN1452
CAS No.:35482-91-8
- TCS 1205
Catalog No.:BCC7819
CAS No.:355022-97-8
- Ki16198
Catalog No.:BCC4560
CAS No.:355025-13-7
- Ki16425
Catalog No.:BCC1155
CAS No.:355025-24-0
- 6'-O-p-Hydroxybenzoylcatalposide
Catalog No.:BCN5297
CAS No.:355143-38-3
- YM-155 hydrochloride
Catalog No.:BCC2066
CAS No.:355406-09-6
Investigation of bendamustine HCL in a phase 2 study in women with resistant ovarian cancer.[Pubmed:22580577]
Invest New Drugs. 2013 Feb;31(1):160-6.
We investigated the safety and efficacy of 90 mg/m(2) Bendamustine HCl, administered intravenously on days 1 and 2 every 28 days in 10 women with platinum and taxane resistant epithelial ovarian cancer. There were no objective tumor responses observed; 2 patients had stable disease. Plasma samples collected at pre-treatment and end of cycle one were analyzed for changes in circulating total cytokeratin 18 and caspase cleaved cytokeratin 18 as exploratory early biomarkers of bendamustine-induced tumor cell death. All patients had measureable levels of both total and cleaved caspase 3 cytokeratin 18, but no relationship with response was possible due to the lack of clinical benefit in treated patients. Due to the high incidence of adverse events and absence of objective responses, only ten patients were treated as predefined by the Simon Two-Stage Design in the protocol. Overall, the regimen was not well tolerated and was associated with fatigue and a greater number of gastrointestinal side effects as compared to previously reported experiences in different patient populations. However, our study subjects did experience less bone marrow suppression. The lack of tolerability could reflect the degree of tumor burden within the peritoneal cavity as well as the high number of prior regimens (median of 5) received by the patients participating in this study.
Phase-I/II study to evaluate dose limiting toxicity, maximum tolerated dose, and tolerability of bendamustine HCl in pre-treated patients with B-chronic lymphocytic leukaemia (Binet stages B and C) requiring therapy.[Pubmed:16292542]
J Cancer Res Clin Oncol. 2006 Feb;132(2):99-104.
PURPOSE: Bendamustine hydrochloride, an anti-neoplastic agent with unique mechanism of action, is known to cause impressive remissions in relapsed non-Hodgkin's lymphoma and chronic lymphocytic leukaemia (CLL). Optimal bendamustine dosing for CLL patients had not been finally established and a phase I/II study was conducted to determine the maximum tolerated dose (MTD) and dose limiting toxicity (DLT) of bendamustine. METHODS: The open-label, single-centre phase I/II study was conducted from March 2001 to September 2002 in Sofia, Bulgaria. The 15 study patients were extendedly pre-treated, but fludarabine-naive (3 female, 12 male, 47-72 years of age, 61 years on average). Bendamustine was given at a starting dose of 100 mg/m2 on days 1 and 2 every 3 weeks based on the previous results in lymphoma. RESULTS: Bendamustine was well tolerated in spite of heavy pre-treatment of the study participants. Toxicity corresponded to the known safety profile of bendamustine, with the exception of bilirubin elevation. The level of 110 mg/m2 was established as MTD. A bendamustine dose of 100 mg/m2 is the recommended dose for further clinical investigations. A 4-week interval is recommended to allow for sufficient recovery. Efficacy results confirmed powerful anti-neoplastic activity of bendamustine even in extendedly pre-treated CLL patients. Based on the remission criteria, nine patients were defined as responders (four CRs, two PR, three NC) and two patients as nonresponders to therapy. Four patients were not evaluable for response, because they had received less than three courses bendamustine. After a follow-up period of 15 months, the four patients with CR were still in remission. One patient with PR had relapsed, the other had ongoing response. CONCLUSIONS: Bendamustine is a very active and well-tolerated drug in patients with pre-treated and refractory CLL. Fludarabine-naivity of patients appears to markedly improve their bendamustine tolerability. First-line use of bendamustine is a safe option for CLL-patients requiring treatment, because bendamustine-owing to its unique pharmacodynamics-(1) is highly effective, (2) reasonably safe, and (3) does hardly produce cross-resistance against other anti-neoplastic drugs effective in this indication.
Bendamustine HCL for the treatment of relapsed indolent non-Hodgkin's lymphoma.[Pubmed:19209254]
Ther Clin Risk Manag. 2008 Aug;4(4):727-32.
Bendamustine is an alkylating agent which also shows properties of a purine analog. Because of its unique mechanism of action it shows activity in relapsed indolent lymphomas which are resistant to alkylating agents, purine analogs, and rituximab. Bendamustine has a favorable toxicity profile causing no alopecia and only a moderate hematotoxicity and gastrointestinal toxicity. Combinations of bendamustine with mitoxantrone and rituximab and with rituximab alone have been shown to be highly active in relapsed/refractory indolent lymphomas and mantle cell lymphomas achieving long lasting complete remissions. Because of only moderate toxicity these combinations can be applied safely in elderly patients who can be treated in an outpatient setting.
Bendamustine therapy in chronic lymphocytic leukemia.[Pubmed:19527193]
Expert Opin Pharmacother. 2009 Jul;10(10):1687-98.
BACKGROUND: Bendamustine is now approved in the US for the treatment of chronic lymphocytic leukemia and low grade non-Hodgkin's lymphoma, and is currently being explored in the treatment of several solid tumor types. OBJECTIVE: The bi-functionality of bendamustine was used to provide a therapeutic understanding of both its benefit as well as adverse effects. METHODS: Pertinent biochemistry and molecular biology pathways are reviewed with regards to bendamustine activity. In view of these pathways bendamustine was reviewed in human clinical trials. RESULTS/CONCLUSION: Bendamustine combines alkylating properties with purine analogue properties making it an effective drug in chronic lymphocytic leukemia where agents that affect these pathways have proven useful. There is limited evidence of cross-reactivity with this agent and other pure purine analogues and alkylators.
In vitro evaluation of bendamustine induced apoptosis in B-chronic lymphocytic leukemia.[Pubmed:12357363]
Leukemia. 2002 Oct;16(10):2096-105.
Bendamustine is a novel cytostatic agent, with activity in non-Hodgkin's lymphomas including B-chronic lymphocytic leukemia (B-CLL). The knowledge about its mode of action, however, is still limited. Here, we investigated the in vitro ability of bendamustine to induce apoptosis on freshly isolated peripheral lymphocytes in B-CLL and analyze the potential underlying mechanisms of action for inducing apoptosis. In CLL cells taken from 37 previously treated and untreated CLL patients, we investigated the influence of bendamustine alone, and in combination with fludarabine, on the induction of apoptosis and changes of Bcl-2 and Bax expression on mRNA and protein level using the RNase protection assay or flow cytometry, respectively. Apoptotic cells were determined with flow cytometry using the fluorescent DNA-binding agent 7-ADD. Using bendamustine alone in concentrations from 1 microg/ml to 50 microg/ml, a dose- and time-dependent manner of cytotoxicity from 30.4% to 94.8% after 48 h could be observed. The LD50 for untreated and pretreated CLL cells was 7.3 or 4.4 microg/ml, respectively. The median apoptotic rate was similar in both groups. The combination of bendamustine with fludarabine led to a highly synergistic effect in inducing apoptosis, which was 150% higher than expected for bendamustine plus fludarabine. The level of the initial Bcl-2 and Bax protein and the m-RNA expression remained unchanged during the incubation with bendamustine. In conclusion, this study demonstrates for the first time the in vitro efficacy of bendamustine in inducing apoptosis in B-CLL cells alone and in combination with fludarabine.


