NorathyriolCAS# 3542-72-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3542-72-1 | SDF | Download SDF |
| PubChem ID | 5281656 | Appearance | Yellow powder |
| Formula | C13H8O6 | M.Wt | 260.2 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
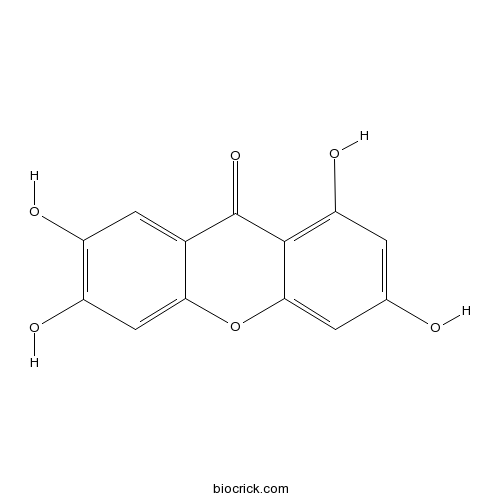
| Chemical Name | 1,3,6,7-tetrahydroxyxanthen-9-one | ||
| SMILES | C1=C(C=C2C(=C1O)C(=O)C3=CC(=C(C=C3O2)O)O)O | ||
| Standard InChIKey | ZHTQCPCDXKMMLU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H8O6/c14-5-1-9(17)12-11(2-5)19-10-4-8(16)7(15)3-6(10)13(12)18/h1-4,14-17H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Norathyriol is a potent PTP1B inhibitor with good cell permeability and oral availability. 2. Norathyriol can improve the glucose utilization and insulin sensitivity by up-regulation of the phosphorylation of AMPK. 3. Norathyriol exerts a potent chemopreventive activity by inhibiting Akt activation in neoplastic cell transformation. 4. Norathyriol as a safe new chemopreventive agent that is highly effective against development of UV-induced skin cancer. 5. Norathyriol may be a dual, yet weak, cyclooxygenase and lipoxygenase pathway blocker, it has anti-inflammatory effect, and has inhibitory effect on the A23187-induced pleurisy and acetic acid-induced writhing response in mice. 6. Norathyriol can relax the rat thoracic aorta mainly by suppressing the Ca2+ influx through both voltage-dependent and receptor-operated calcium channels. |
| Targets | PKB | Akt | AMPK | AP-1 | ERK | NF-kB | PGE | COX | Calcium Channel | Potassium Channel | cAMP |

Norathyriol Dilution Calculator

Norathyriol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8432 mL | 19.216 mL | 38.432 mL | 76.864 mL | 96.0799 mL |
| 5 mM | 0.7686 mL | 3.8432 mL | 7.6864 mL | 15.3728 mL | 19.216 mL |
| 10 mM | 0.3843 mL | 1.9216 mL | 3.8432 mL | 7.6864 mL | 9.608 mL |
| 50 mM | 0.0769 mL | 0.3843 mL | 0.7686 mL | 1.5373 mL | 1.9216 mL |
| 100 mM | 0.0384 mL | 0.1922 mL | 0.3843 mL | 0.7686 mL | 0.9608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- J 113863
Catalog No.:BCC7422
CAS No.:353791-85-2
- Honokiol
Catalog No.:BCN1001
CAS No.:35354-74-6
- HLM006474
Catalog No.:BCC5403
CAS No.:353519-63-8
- 9-Hydroxycalabaxanthone
Catalog No.:BCN5293
CAS No.:35349-68-9
- TQS
Catalog No.:BCC7896
CAS No.:353483-92-8
- Monocrotaline N-oxide
Catalog No.:BCN2097
CAS No.:35337-98-5
- S26948
Catalog No.:BCC7751
CAS No.:353280-43-0
- NS 6180
Catalog No.:BCC6307
CAS No.:353262-04-1
- LY 487379 hydrochloride
Catalog No.:BCC7627
CAS No.:353229-59-1
- D-Alaninol
Catalog No.:BCC2728
CAS No.:35320-23-1
- (E)-3-Hydroxy-5-methoxystilbene
Catalog No.:BCN5292
CAS No.:35302-70-6
- Ziyuglycoside II
Catalog No.:BCN5291
CAS No.:35286-59-0
- 1-Methyl-5-nitro-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8470
CAS No.:3543-72-4
- 1-Methyl-5-amino-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8469
CAS No.:3543-73-5
- 5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8724
CAS No.:3543-74-6
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- INO-1001
Catalog No.:BCC2212
CAS No.:3544-24-9
- Caraphenol A
Catalog No.:BCN5295
CAS No.:354553-35-8
- Hirsuteine
Catalog No.:BCN2756
CAS No.:35467-43-7
- Corianin
Catalog No.:BCN5296
CAS No.:35481-77-7
- SC-514
Catalog No.:BCC4554
CAS No.:354812-17-2
- Balicatib
Catalog No.:BCC5139
CAS No.:354813-19-7
- 3-beta-O-(trans-p-Coumaroyl)maslinic acid
Catalog No.:BCN1452
CAS No.:35482-91-8
- TCS 1205
Catalog No.:BCC7819
CAS No.:355022-97-8
Norathyriol reverses obesity- and high-fat-diet-induced insulin resistance in mice through inhibition of PTP1B.[Pubmed:24985145]
Diabetologia. 2014 Oct;57(10):2145-54.
AIM/HYPOTHESIS: Protein tyrosine phosphatase 1B (PTP1B) negatively regulates insulin signalling. PTP1B deficiency improves obesity-induced insulin resistance and consequently improves type 2 diabetes in mice. Here, the small molecule Norathyriol reversed obesity- and high-fat-diet-induced insulin resistance by inhibiting PTP1B. METHODS: The inhibitory mode of PTP1B was evaluated by using the double-reciprocal substrate in the presence of Norathyriol. Primary cultured hepatocytes, myoblasts and white adipocytes were used to investigate the effect of Norathyriol on insulin signalling. Glucose homeostasis and insulin sensitivity were characterised by glucose and insulin tolerance tests. RESULTS: Norathyriol was identified as a competitive inhibitor of PTP1B, with an IC50 of 9.59 +/- 0.39 mumol/l. In cultured hepatocytes and myoblasts, Norathyriol treatment blocked the PTP1B-mediated dephosphorylation of the insulin receptor. Intraperitoneal injection of Norathyriol inhibited liver and muscle PTP1B activity in mice, thus contributing to the improved glucose homeostasis and insulin sensitivity. However, these beneficial effects were abolished in PTP1B-deficient mice. Notably, oral administration of Norathyriol protected mice from diet-induced obesity and insulin resistance through inhibition of hypothalamic PTP1B activity. CONCLUSIONS/INTERPRETATION: Our results indicate that the small molecule Norathyriol is a potent PTP1B inhibitor with good cell permeability and oral availability.
Norathyriol suppresses transformation in JB6 P+ cells by the inhibition of Akt.[Pubmed:23361275]
J Cancer Res Ther. 2012 Oct-Dec;8(4):561-4.
CONTEXT: Chemoprevention has been acknowledged as an important and practical strategy for the management of skin cancer. Norathyriol, a naturally occurring compound present in various plants, has a potent anticancer-promoting activity. AIMS: The aim was to investigate the chemopreventive activity of Norathyriol on JB6 P+ cells. MATERIALS AND METHODS: A soft agar assay was used to detect the effect of Norathyriol on cell transformation. The activator protein-1 (AP-1) transactivation activity was examined by the luciferase assay. RESULTS: Norathyriol inhibited epidermal growth factor (EGF)- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced neoplastic cell transformation in a dose-dependent manner. The activation of activator protein-1 was dose dependently suppressed by Norathyriol treatment. Western blot data revealed that Norathyriol attenuated the phosphorylation of Akt. CONCLUSIONS: Norathyriol exerts a potent chemopreventive activity by inhibiting Akt activation in neoplastic cell transformation.
Effect of norathyriol, isolated from Tripterospermum lanceolatum, on A23187-induced pleurisy and analgesia in mice.[Pubmed:7935860]
Naunyn Schmiedebergs Arch Pharmacol. 1994 Jul;350(1):90-5.
A23187-induced pleurisy in the mouse was demonstrated in this study. The protein leakage, leukocyte accumulation, LTB4 and PGE2 production in the pleural cavity of mice were increased by A23187 in a dose-dependent manner. At 7.5 nmole A23187 intrapleural injection, the protein level peaked at 0.5-2 h, PMN leukocytes accumulation peaked at 3-4 h, and LTB4 and PGE2 production peaked at 0.5-1 h. In this in vivo model we investigated the anti-inflammatory effect of Norathyriol, isolated from Tripterospermum lanceolatum. A23187-induced protein leakage was reduced by Norathyriol (ID50 was about 30.6 mg/kg i.p.), indomethacin and BW755C. A23187-induced PMN leukocytes accumulation was suppressed by Norathyriol (ID50 was about 16.8 mg/kg, i.p.) and BW755C, while enhanced by indomethacin. Like BW755C, Norathyriol reduced both LTB4 and PGE2 production (ID50 was about 18.6 and 29.1 mg/kg i.p., respectively), while indomethacin reduced PGE2 but not LTB4 generation. We also demonstrated the analgesic effect of Norathyriol on the acetic acid-induced writhing response. Acetic acid-induced writhing response was depressed by Norathyriol (ID50 was about 27.9 mg/kg i.p.), indomethacin and ibuprofen. These results suggest that Norathyriol, like BW755C, might be a dual, yet weak, cyclooxygenase and lipoxygenase pathway blocker. The inhibitory effect of Norathyriol on the A23187-induced pleurisy and acetic acid-induced writhing response in mice is proposed to be dependent on the reduction of eicosanoids mediators formation in the inflammatory site.
Mangiferin and its aglycone, norathyriol, improve glucose metabolism by activation of AMP-activated protein kinase.[Pubmed:24033319]
Pharm Biol. 2014 Jan;52(1):68-73.
CONTEXT: Mangiferin has been reported to possess antidiabetic activities. Norathyriol, a xanthone aglycone, has the same structure as mangiferin, except for a C-glucosyl bond. To our best knowledge, no study has been conducted to determine and compare those two compounds on glucose consumption in vitro. OBJECTIVE: In this study, the effects of Norathyriol and mangiferin on glucose consumption in normal and insulin resistance (IR) L6 myotubes were evaluated. Simultaneously, the potential mechanism of this effect was also investigated. MATERIALS AND METHODS: Normal or IR L6 myotubes were incubated with Norathyriol (2.5 approximately 10 muM, 0.625 approximately 2.5 muM), mangiferin (10 approximately 40 muM, 2.5 approximately 10 muM) or rosiglitazone (20 muM) and/or 0.05 nM insulin for 24 h, respectively. The glucose consumption was assessed using the glucose oxidase method. Immunoblotting was performed to detect protein kinase B (PKB/Akt) and AMP-activated protein kinase (AMPK) phosphorylation in L6 myotubes cells. RESULTS: Norathyriol and mangiferin treatment alone increased the glucose consumption 61.9 and 56.3%, respectively, in L6 myotubes and made additional increasing with 0.05 nM insulin. In IR L6 myotubes, Norathyriol treatment made increasing with or without insulin, mangiferin treatment also made increasing but only when co-treated with insulin. Immunoblotting results showed that Norathyriol and mangiferin produced an increase of 1.9 - and 1.8-fold in the phosphorylation levels of the AMPK, but not in Akt. DISCUSSION AND CONCLUSION: Our findings suggest that Norathyriol and mangiferin could improve the glucose utilization and insulin sensitivity by up-regulation of the phosphorylation of AMPK. Norathyriol may be considered as an active metabolite responsible for the antidiabetic activity of mangiferin.
Host-guest inclusion system of norathyriol with beta-cyclodextrin and its derivatives: preparation, characterization, and anticancer activity.[Pubmed:24508024]
J Biosci Bioeng. 2014 Jun;117(6):775-9.
The characterization, binding ability and inclusion complexation behavior of the inclusion complexes of Norathyriol with beta-cyclodextrin (beta-CD) and its derivatives such as hydroxypropyl-beta-cyclodextrin (HPbetaCD), sulfobutyl ether beta-cyclodextrin (SBEbetaCD) and mono (6-ethylene-diamino-6-deoxy)-beta-cyclodextrin (ENbetaCD) were investigated in both solution and solid state by means of femtosecond spectroscopy, (1)H and 2D nuclear magnetic resonance, powder X-ray diffraction. The results showed that the aqueous solubility of the complexes was much higher than that of Norathyriol. The cytotoxicity of complexes on human colon cancer cell lines HT-29, SW480, Lovo and HCT116 indicated that the antitumor activities of the complexes were better than that of Norathyriol. This high antitumor activity, along with the satisfactory aqueous solubility of the complexes, will be potentially useful for their application on cancer chemotherapies.
Vasorelaxation of rat thoracic aorta caused by norathyriol isolated from Gentianaceae.[Pubmed:1645671]
Eur J Pharmacol. 1991 Jan 3;192(1):133-9.
The pharmacological effects of Norathyriol on isolated rat thoracic aorta were examined. In the high-K+ (60 mM) medium, Ca2+ (0.03 to 3 mM)-induced vasocontraction was inhibited concentration dependently by Norathyriol. Given as pretreatment Norathyriol (20 to 200 microM) also inhibited the norepinephrine (NE, 3 microM)-induced tonic contraction. However, the phasic contraction was inhibited only by high concentrations of Norathyriol (200 and 400 microM). The tonic contraction elicited by NE was also relaxed by the addition of Norathyriol. This relaxing effect of Norathyriol was not antagonized by methylene blue (50 microM) or indomethacin (20 microM) and was still seen in denuded rat aorta. Although the cAMP level was not changed by Norathyriol, the cGMP level was increased by a high concentration of Norathyriol (400 microM). [3H]Inositol monophosphate formation caused by NE was not affected by Norathyriol at concentration of either 100 or 400 microM. The 45Ca2+ influx caused by either NE or high K+ was inhibited by Norathyriol in a concentration-dependent manner. It is concluded that Norathyriol relaxed the rat thoracic aorta mainly by suppressing the Ca2+ influx through both voltage-dependent and receptor-operated calcium channels.
Norathyriol suppresses skin cancers induced by solar ultraviolet radiation by targeting ERK kinases.[Pubmed:22084399]
Cancer Res. 2012 Jan 1;72(1):260-70.
Ultraviolet (UV) irradiation is the leading factor in the development of skin cancer, prompting great interest in chemopreventive agents for this disease. In this study, we report the discovery of Norathyriol, a plant-derived chemopreventive compound identified through an in silico virtual screening of the Chinese Medicine Library. Norathyriol is a metabolite of mangiferin found in mango, Hypericum elegans, and Tripterospermum lanceolatum and is known to have anticancer activity. Mechanistic investigations determined that Norathyriol acted as an inhibitor of extracellular signal-regulated kinase (ERK)1/2 activity to attenuate UVB-induced phosphorylation in mitogen-activated protein kinases signaling cascades. We confirmed the direct and specific binding of Norathyriol with ERK2 through a cocrystal structural analysis. The xanthone moiety in Norathyriol acted as an adenine mimetic to anchor the compound by hydrogen bonds to the hinge region of the protein ATP-binding site on ERK2. Norathyriol inhibited in vitro cell growth in mouse skin epidermal JB6 P+ cells at the level of G(2)-M phase arrest. In mouse skin tumorigenesis assays, Norathyriol significantly suppressed solar UV-induced skin carcinogenesis. Further analysis indicated that Norathyriol mediates its chemopreventive activity by inhibiting the ERK-dependent activity of transcriptional factors AP-1 and NF-kappaB during UV-induced skin carcinogenesis. Taken together, our results identify Norathyriol as a safe new chemopreventive agent that is highly effective against development of UV-induced skin cancer.


