9-HydroxycalabaxanthoneCAS# 35349-68-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 35349-68-9 | SDF | Download SDF |
| PubChem ID | 5495929 | Appearance | Yellow powder |
| Formula | C24H24O6 | M.Wt | 408.5 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
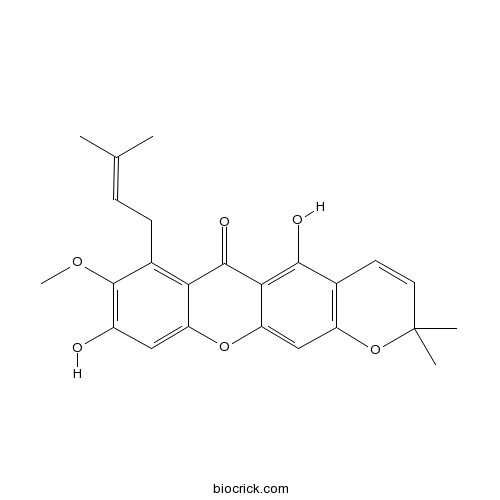
| Chemical Name | 5,9-dihydroxy-8-methoxy-2,2-dimethyl-7-(3-methylbut-2-enyl)pyrano[3,2-b]xanthen-6-one | ||
| SMILES | CC(=CCC1=C2C(=CC(=C1OC)O)OC3=CC4=C(C=CC(O4)(C)C)C(=C3C2=O)O)C | ||
| Standard InChIKey | HQWHKELJHUFLGD-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 9-Hydroxycalabaxanthone exhibits cytotoxicity against the HT-29 cell line with ED50 values of 9.1 microM. The combination of 9-hydroxycalabaxanthone with α-mangostin shows the synergistic antimalarial interaction in both clones. |
| Targets | Antifection |
| In vitro | Mangostanaxanthones I and II, new xanthones from the pericarp of Garcinia mangostana.[Pubmed: 25128900 ]Fitoterapia. 2014 Oct;98:215-21.Two new xanthones: mangostanaxanthones I (3) and II (5) were isolated from the pericarp of Garcinia mangostana, along with four known xanthones: 9-Hydroxycalabaxanthone (1), parvifolixanthone C (2), α-mangostin (4), and rubraxanthone (6). The in vitro antimalarial interaction of 9-hydroxycalabaxanthone and α-mangostin with mefloquine/artesunate.[Pubmed: 26204026]Acta Parasitol. 2014 Mar;60(1):105-11.Multidrug resistance Plasmodium falciparum is the major health problem in Thailand. Discovery and development of new antimalarial drugs with novel modes of action is urgently required. The aim of the present study was to investigate the antimalarial interaction of 9-Hydroxycalabaxanthone and α-mangostin with the standard antimalarial drugs mefloquine and artesunate in chloroquine sensitive (3D7) and chloroquine resistant (K1) P. falciparum clones in vitro. |
| Structure Identification | J Nat Prod. 2009 Nov;72(11):2028-31.Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen).[Pubmed: 19839614]
|

9-Hydroxycalabaxanthone Dilution Calculator

9-Hydroxycalabaxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.448 mL | 12.2399 mL | 24.4798 mL | 48.9596 mL | 61.1995 mL |
| 5 mM | 0.4896 mL | 2.448 mL | 4.896 mL | 9.7919 mL | 12.2399 mL |
| 10 mM | 0.2448 mL | 1.224 mL | 2.448 mL | 4.896 mL | 6.12 mL |
| 50 mM | 0.049 mL | 0.2448 mL | 0.4896 mL | 0.9792 mL | 1.224 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2448 mL | 0.4896 mL | 0.612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TQS
Catalog No.:BCC7896
CAS No.:353483-92-8
- Monocrotaline N-oxide
Catalog No.:BCN2097
CAS No.:35337-98-5
- S26948
Catalog No.:BCC7751
CAS No.:353280-43-0
- NS 6180
Catalog No.:BCC6307
CAS No.:353262-04-1
- LY 487379 hydrochloride
Catalog No.:BCC7627
CAS No.:353229-59-1
- D-Alaninol
Catalog No.:BCC2728
CAS No.:35320-23-1
- (E)-3-Hydroxy-5-methoxystilbene
Catalog No.:BCN5292
CAS No.:35302-70-6
- Ziyuglycoside II
Catalog No.:BCN5291
CAS No.:35286-59-0
- Ziyuglycoside I
Catalog No.:BCN5290
CAS No.:35286-58-9
- 1-Chloroindan
Catalog No.:BCN2244
CAS No.:35275-62-8
- Caesalmin B
Catalog No.:BCN7252
CAS No.:352658-23-2
- Delafloxacin meglumine
Catalog No.:BCC1523
CAS No.:352458-37-8
- HLM006474
Catalog No.:BCC5403
CAS No.:353519-63-8
- Honokiol
Catalog No.:BCN1001
CAS No.:35354-74-6
- J 113863
Catalog No.:BCC7422
CAS No.:353791-85-2
- Norathyriol
Catalog No.:BCN5294
CAS No.:3542-72-1
- 1-Methyl-5-nitro-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8470
CAS No.:3543-72-4
- 1-Methyl-5-amino-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8469
CAS No.:3543-73-5
- 5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8724
CAS No.:3543-74-6
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- INO-1001
Catalog No.:BCC2212
CAS No.:3544-24-9
- Caraphenol A
Catalog No.:BCN5295
CAS No.:354553-35-8
- Hirsuteine
Catalog No.:BCN2756
CAS No.:35467-43-7
- Corianin
Catalog No.:BCN5296
CAS No.:35481-77-7
Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen).[Pubmed:19839614]
J Nat Prod. 2009 Nov;72(11):2028-31.
Bioassay-guided fractionation of a chloroform-soluble extract of Garcinia mangostana stem bark, using the HT-29 human colon cancer cell line and an enzyme-based ELISA NF-kappaB assay, led to the isolation of a new xanthone, 11-hydroxy-3-O-methyl-1-isomangostin (1). The structure of 1 was elucidated by spectroscopic data analysis. In addition, 10 other known compounds, 11-hydroxy-1-isomangostin (2), 11alpha-mangostanin (3), 3-isomangostin (4), alpha-mangostin (5), beta-mangostin (6), garcinone D (7), 9-Hydroxycalabaxanthone (8), 8-deoxygartanin (9), gartanin (10), and cratoxyxanthone (11), were isolated. Compounds 4-8 exhibited cytotoxicity against the HT-29 cell line with ED50 values of 4.9, 1.7, 1.7, 2.3, and 9.1 microM, respectively. In an ELISA NF-kappaB assay, compounds 5-7, 9, and 10 inhibited p65 activation with IC50 values of 15.9, 12.1, 3.2, 11.3, and 19.0 microM, respectively, and 6 showed p50 inhibitory activity with an IC50 value of 7.5 microM. Alpha-mangostin (5) was further tested in an in vivo hollow fiber assay, using HT-29, LNCaP, and MCF-7 cells, but it was found to be inactive at the highest dose tested (20 mg/kg).
The in vitro antimalarial interaction of 9-hydroxycalabaxanthone and alpha-mangostin with mefloquine/artesunate.[Pubmed:26204026]
Acta Parasitol. 2014 Mar;60(1):105-11.
Multidrug resistance Plasmodium falciparum is the major health problem in Thailand. Discovery and development of new antimalarial drugs with novel modes of action is urgently required. The aim of the present study was to investigate the antimalarial interaction of 9-Hydroxycalabaxanthone and alpha-mangostin with the standard antimalarial drugs mefloquine and artesunate in chloroquine sensitive (3D7) and chloroquine resistant (K1) P. falciparum clones in vitro. Median (range) IC50 (drug concentration which produces 50% parasite growth inhibition) values of the 9-Hydroxycalabaxanthone, alpha-mangostin, artesunate and mefloquine for 3D7 vs K1 clones were 1.5 (0.9-2.1) vs 1.2 (1.1-1.6) muM, 17.9 (15.7.0-20.0) vs 9.7 (6.0-14.0) muM, 1.0 (0.4-3.0) vs 1.7 (1.0-2.5) nM, and 13.3 (11.1-13.3) vs 7.1 (6.7-12.2) nM, respectively. Analysis of isobologram and combination index (CI) of 9-Hydroxycalabaxanthone with artesunate or mefloquine showed synergistic and indifference antimalarial interaction, respectively. alpha-mangostin-artesunate combination exhibited a slight antagonistic effect of antimalarial interaction, whereas alpha-mangostin and mefloquine combination showed indifference interaction in both clones. The combination of 9-Hydroxycalabaxanthone with alpha-mangostin showed the synergistic antimalarial interaction in both clones.
Mangostanaxanthones I and II, new xanthones from the pericarp of Garcinia mangostana.[Pubmed:25128900]
Fitoterapia. 2014 Oct;98:215-21.
Two new xanthones: mangostanaxanthones I (3) and II (5) were isolated from the pericarp of Garcinia mangostana, along with four known xanthones: 9-Hydroxycalabaxanthone (1), parvifolixanthone C (2), alpha-mangostin (4), and rubraxanthone (6). Their structures were elucidated on the basis of IR, UV, 1D, 2D NMR, and MS spectroscopic data, in addition to comparison with literature data. The isolated compounds were evaluated for their antioxidant, antimicrobial, and quorum-sensing inhibitory activities. Compounds 3 and 5 displayed promising antioxidant activity with IC50 12.07 and 14.12 muM, respectively using DPPH assay. Compounds 4-6 had weak to moderate activity against Escherichia coli and Staphylococcus aureus, while demonstrated promising action against Bacillus cereus with MICs 0.25, 1.0, and 1.0mg/mL, respectively. The tested compounds were inactive against Candida albicans. However, they showed selective antifungal potential toward Aspergillus fumigatus. Compounds 3 and 4 possessed quorum-sensing inhibitory activity against Chromobacterium violaceum ATCC 12472.


