Ki16425LPA receptor antagonist CAS# 355025-24-0 |
- AM966
Catalog No.:BCC1355
CAS No.:1228690-19-4
- AM-095 free base
Catalog No.:BCC1352
CAS No.:1228690-36-5
- AM095
Catalog No.:BCC1351
CAS No.:1345614-59-6
- Ki16198
Catalog No.:BCC4560
CAS No.:355025-13-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 355025-24-0 | SDF | Download SDF |
| PubChem ID | 10367662 | Appearance | Powder |
| Formula | C23H23ClN2O5S | M.Wt | 474.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (210.54 mM) *"≥" means soluble, but saturation unknown. | ||
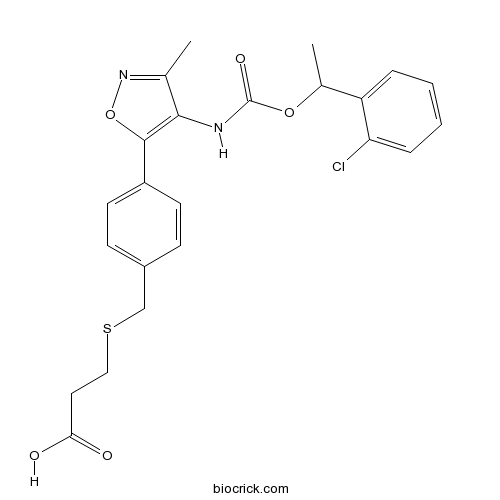
| Chemical Name | 3-[[4-[4-[1-(2-chlorophenyl)ethoxycarbonylamino]-3-methyl-1,2-oxazol-5-yl]phenyl]methylsulfanyl]propanoic acid | ||
| SMILES | CC1=NOC(=C1NC(=O)OC(C)C2=CC=CC=C2Cl)C3=CC=C(C=C3)CSCCC(=O)O | ||
| Standard InChIKey | LLIFMNUXGDHTRO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H23ClN2O5S/c1-14-21(25-23(29)30-15(2)18-5-3-4-6-19(18)24)22(31-26-14)17-9-7-16(8-10-17)13-32-12-11-20(27)28/h3-10,15H,11-13H2,1-2H3,(H,25,29)(H,27,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antagonist of the lysophosphatidic acid receptors LPA1 and LPA3 (Ki values are 0.25 and 0.36 μM respectively, in a GTPγS binding assay). Blocks LPA-induced dephosphorylation of Yes-associated protein (YAP) and WW domain-containing transcription regulator protein 1 (TAZ), inhibiting the Hippo signaling pathway, in HEK293A cells. |

Ki16425 Dilution Calculator

Ki16425 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1054 mL | 10.5272 mL | 21.0544 mL | 42.1088 mL | 52.636 mL |
| 5 mM | 0.4211 mL | 2.1054 mL | 4.2109 mL | 8.4218 mL | 10.5272 mL |
| 10 mM | 0.2105 mL | 1.0527 mL | 2.1054 mL | 4.2109 mL | 5.2636 mL |
| 50 mM | 0.0421 mL | 0.2105 mL | 0.4211 mL | 0.8422 mL | 1.0527 mL |
| 100 mM | 0.0211 mL | 0.1053 mL | 0.2105 mL | 0.4211 mL | 0.5264 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ki16425 is a subtype-selective antagonist of lysophosphatidic acid receptor (LPA) with Ki values of 0.34 μM for LPA1, 6.5μM for LPA2, and 0.93μM for LPA3 [4].
It has been reported that Ki16425 inhibited the LPA receptor-mediated actions in the order of LPA1≥LPA3≥LPA2. Ki16425 inhibited LPA-induced guanosine 5’-O-(3-thio) triphosphate binding, membrane fractions binding, and was involved in the long-term responses, including DNA synthesis and cell migration [1]. Ki16425 attenuated LPA-mediated intracellular signaling and invasion responses in vitro. Co-treatment of Ki16425 and sunitinib prolonged the sensitivity of renal tumor cell to sunitinib in xenograft mouse models [3]. In human breast cancer cells, Ki16425 can inhibit heparin-binding EGF-like growth factor (HB-EGF) expression [4].
In mouse thoracic aorta, Ki16425 significantly reduced LPA-induced vasorelaxation [2]. Ki16425 treatment also blocked renal tumorigenesis in vivo [3]. A five-day treatment with Ki16425 significantly decreased both HB-EGF mRNA expression at the transplanted tumor site in mice and circulating human HB-EGF concentrations in serum [4].
References:
[1]. Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, Yagi M, Sato M, Suzuki R, Murooka H, Sakai T, Nishitoba T, Im DS, Nochi H, Tamoto K, Tomura H, Okajima F. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003 Oct;64(4):994-1005.
[2]. Ruisanchez É, Dancs P, Kerék M, Németh T, Faragó B, Balogh A, Patil R, Jennings BL, Liliom K, Malik KU, Smrcka AV, Tigyi G, Benyó Z. Lysophosphatidic acid induces vasodilation mediated by LPA1 receptors, phospholipase C, and endothelial nitric oxide synthase. FASEB J. 2014 Feb;28(2):880-90.
[3]. Su SC, Hu X, Kenney PA, Merrill MM, Babaian KN, Zhang XY, Maity T, Yang SF, Lin X, Wood CG. Autotaxin-lysophosphatidic acid signaling axis mediates tumorigenesis and development of acquired resistance to sunitinib in renal cell carcinoma. Clin Cancer Res. 2013 Dec 1;19(23):6461-72.
[4]. David M, Sahay D, Mege F, Descotes F, Leblanc R, Ribeiro J, Clézardin P, Peyruchaud O. Identification of Heparin-Binding EGF-Like Growth Factor (HB-EGF) as a Biomarker for Lysophosphatidic Acid Receptor Type 1 (LPA1) Activation in Human Breast and Prostate Cancers. PLoS One. 2014 May 14;9(5):e97771.
- Ki16198
Catalog No.:BCC4560
CAS No.:355025-13-7
- TCS 1205
Catalog No.:BCC7819
CAS No.:355022-97-8
- 3-beta-O-(trans-p-Coumaroyl)maslinic acid
Catalog No.:BCN1452
CAS No.:35482-91-8
- Balicatib
Catalog No.:BCC5139
CAS No.:354813-19-7
- SC-514
Catalog No.:BCC4554
CAS No.:354812-17-2
- Corianin
Catalog No.:BCN5296
CAS No.:35481-77-7
- Hirsuteine
Catalog No.:BCN2756
CAS No.:35467-43-7
- Caraphenol A
Catalog No.:BCN5295
CAS No.:354553-35-8
- INO-1001
Catalog No.:BCC2212
CAS No.:3544-24-9
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- 5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8724
CAS No.:3543-74-6
- 1-Methyl-5-amino-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8469
CAS No.:3543-73-5
- 6'-O-p-Hydroxybenzoylcatalposide
Catalog No.:BCN5297
CAS No.:355143-38-3
- YM-155 hydrochloride
Catalog No.:BCC2066
CAS No.:355406-09-6
- Buflomedil HCl
Catalog No.:BCC4760
CAS No.:35543-24-9
- N,N-dimethyl-2-Quinoxalinamine
Catalog No.:BCC9066
CAS No.:35552-76-2
- tert-Butyl rosuvastatin
Catalog No.:BCC9163
CAS No.:355806-00-7
- Betmidin
Catalog No.:BCN8253
CAS No.:35589-22-1
- Fluocinonide
Catalog No.:BCC4953
CAS No.:356-12-7
- Darapladib
Catalog No.:BCC1515
CAS No.:356057-34-6
- Toceranib
Catalog No.:BCC2005
CAS No.:356068-94-5
- N-Desethyl Sunitinib
Catalog No.:BCC1792
CAS No.:356068-97-8
- CP 20961
Catalog No.:BCC6063
CAS No.:35607-20-6
- Mesuaxanthone A
Catalog No.:BCN5298
CAS No.:3561-81-7
Protean agonism of the lysophosphatidic acid receptor-1 with Ki16425 reduces nerve growth factor-induced neurite outgrowth in pheochromocytoma 12 cells.[Pubmed:16945108]
J Neurochem. 2006 Sep;98(6):1920-9.
We report here a novel role for the constitutively active lysophosphatidic acid receptor-1 (LPA(1)) receptor in providing Gbetagamma subunits for use by the Trk A receptor. This enhances the ability of nerve growth factor (NGF) to promote signalling and cell response. These conclusions were based on three lines of evidence. Firstly, the LPA(1) receptor was co-immunoprecipitated with the Trk A receptor from lysates, suggesting that these proteins form a complex. Secondly, Ki16425, a selective protean agonist of the LPA(1) receptor, decreased constitutive basal and LPA-induced LPA(1) receptor-stimulated GTPgammaS binding. Ki16425 reduced the LPA-induced activation of p42/p44 mitogen activated protein kinase (MAPK), while acting as a weak stimulator of p42/p44 MAPK on its own, properties typical of a protean agonist. Significantly, Ki16425 also reduced the NGF-induced stimulation of p42/p44 MAPK and inhibited NGF-stimulated neurite outgrowth. Thirdly, the over-expression of the C-terminal GRK-2 peptide, which sequesters Gbetagamma subunits, reduced the NGF-induced activation of p42/p44 MAPK. In contrast, the stimulation of PC12 cells with LPA leads to a predominant G(i)alpha2-mediated Trk A-independent activation of p42/p44 MAPK, where Gbetagamma subunits play a diminished role. These findings suggest a novel role for the constitutively active LPA(1) receptor in regulating NGF-induced neuronal differentiation.
Lysophosphatidic acid receptor 1 antagonist ki16425 blunts abdominal and systemic inflammation in a mouse model of peritoneal sepsis.[Pubmed:25701366]
Transl Res. 2015 Jul;166(1):80-8.
Lysophosphatidic acid (LPA) is a bioactive lipid mediator of inflammation via the LPA receptors 1-6. We and others have previously described proinflammatory and profibrotic activities of LPA signaling in bleomycin- or lipopolysaccharide (LPS)-induced pulmonary fibrosis or lung injury models. In this study, we investigated if LPA signaling plays a role in the pathogenesis of systemic sepsis from an abdominal source. We report here that antagonism of the LPA receptor LPA1 with the small molecule Ki16425 reduces the severity of abdominal inflammation and organ damage in the setting of peritoneal endotoxin exposure. Pretreatment of mice with intraperitoneal Ki16425 eliminates LPS-induced peritoneal neutrophil chemokine and cytokine production, liver oxidative stress, liver injury, and cellular apoptosis in visceral organs. Mice pretreated with Ki16425 are also protected from LPS-induced mortality. Tissue myeloperoxidase activity is not affected by LPA1 antagonism. We have shown that LPA1 is associated with LPS coreceptor CD14 and the association is suppressed by Ki16425. LPS-induced phosphorylation of protein kinase C delta (PKCdelta) and p38 mitogen-activated protein kinase (p38 MAPK) in liver cells and interleukin 6 production in Raw264 cells are likewise blunted by LPA1 antagonism. These studies indicate that the small molecule inhibitor of LPA1, Ki16425, suppresses cytokine responses and inflammation in a peritoneal sepsis model by blunting downstream signaling through the LPA1-CD14-toll-like receptor 4 receptor complex. This anti-inflammatory effect may represent a therapeutic strategy for the treatment of systemic inflammatory responses to infection of the abdominal cavity.
Lysophosphatidylserine induces calcium signaling through Ki16425/VPC32183-sensitive GPCR in bone marrow-derived mast cells and in C6 glioma and colon cancer cells.[Pubmed:18409043]
Arch Pharm Res. 2008 Mar;31(3):310-7.
Lysophosphatidylserine (LPS) can be generated following phosphatidylserine-specific phospholipase A2 activation. The effects of LPS on cellular activities and the identities of its target molecules, however, have not been fully elucidated. In this study, we observed that LPS stimulated intracellular calcium increased in mouse bone marrow-derived mast cells (BMMC), and rat C6 glioma and human HCT116 colon cancer cells and compared the LPS-induced Ca2+ increases with the response by lysophosphatidic acid (LPA), a structurally related bioactive lysolipid. In order to test involvement of signaling molecules in the LPS-induced Ca2+ signaling, we used pertussis toxin (PTX), U73122, and 2-APB, which are specific inhibitors for G proteins, phospholipase C (PLC), and IP3 receptors, respectively. The increases due to LPS and LPA were inhibited by PTX, U-73122 and 2-APB, suggesting that both lipids stimulate calcium signaling via G proteins (Gi/o types), PLC activation, and subsequent IP3 production, although the sensitivity to pharmacological inhibitors varied from complete inhibition to partial inhibition depending on cell type and lysolipid. Furthermore, we observed that Ki16425 completely inhibited an LPS-induced Ca2+ response in three cell types, but that the effect of VPC32183 varied from complete inhibition in BMMC and C6 glioma cells to partial inhibition in HCT116 cells. Therefore, we conclude that LPS increases [Ca2+]i through Ki16425/VPC32183-sensitive G protein-coupled receptors (GPCR), G protein, PLC, and IP3 in mouse BMMC, rat C6, and human HCT116 cells.
Synthesis and biological evaluation of optically active Ki16425.[Pubmed:22658556]
Bioorg Med Chem Lett. 2012 Jul 1;22(13):4323-6.
An enantionselective synthesis of both enantiomers of Ki16425, which possesses selective LPA antagonistic activity, was achieved. The isoxazole core was constructed by a 1,3-dipolar cycloaddition of nitrile oxide with alkyne and condensation with the optically active alpha-phenethyl alcohol segment, which was prepared by an enantioselective reduction of arylmethylketone. Biological evaluation of both enantiomers of Ki16425 revealed that the (R)-isomer showed much higher antagonistic activity for LPA(1) and LPA(3) receptors.
Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling.[Pubmed:22863277]
Cell. 2012 Aug 17;150(4):780-91.
The Hippo pathway is crucial in organ size control, and its dysregulation contributes to tumorigenesis. However, upstream signals that regulate the mammalian Hippo pathway have remained elusive. Here, we report that the Hippo pathway is regulated by G-protein-coupled receptor (GPCR) signaling. Serum-borne lysophosphatidic acid (LPA) and sphingosine 1-phosphophate (S1P) act through G12/13-coupled receptors to inhibit the Hippo pathway kinases Lats1/2, thereby activating YAP and TAZ transcription coactivators, which are oncoproteins repressed by Lats1/2. YAP and TAZ are involved in LPA-induced gene expression, cell migration, and proliferation. In contrast, stimulation of Gs-coupled receptors by glucagon or epinephrine activates Lats1/2 kinase activity, thereby inhibiting YAP function. Thus, GPCR signaling can either activate or inhibit the Hippo-YAP pathway depending on the coupled G protein. Our study identifies extracellular diffusible signals that modulate the Hippo pathway and also establishes the Hippo-YAP pathway as a critical signaling branch downstream of GPCR.
Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1.[Pubmed:14660630]
J Biol Chem. 2004 Feb 20;279(8):6595-605.
Cytokines and growth factors in malignant ascites are thought to modulate a variety of cellular activities of cancer cells and normal host cells. The motility of cancer cells is an especially important activity for invasion and metastasis. Here, we examined the components in ascites, which are responsible for cell motility, from patients and cancer cell-injected mice. Ascites remarkably stimulated the migration of pancreatic cancer cells. This response was inhibited or abolished by pertussis toxin, monoglyceride lipase, an enzyme hydrolyzing lysophosphatidic acid (LPA), and Ki16425 and VPC12249, antagonists for LPA receptors (LPA1 and LPA3), but not by an LPA3-selective antagonist. These agents also inhibited the response to LPA but not to the epidermal growth factor. In malignant ascites, LPA is present at a high level, which can explain the migration activity, and the fractionation study of ascites by lipid extraction and subsequent thin-layer chromatography indicated LPA as an active component. A significant level of LPA1 receptor mRNA is expressed in pancreatic cancer cells with high migration activity to ascites but not in cells with low migration activity. Small interfering RNA against LPA1 receptors specifically inhibited the receptor mRNA expression and abolished the migration response to ascites. These results suggest that LPA is a critical component of ascites for the motility of pancreatic cancer cells and LPA1 receptors may mediate this activity. LPA receptor antagonists including Ki16425 are potential therapeutic drugs against the migration and invasion of cancer cells.
Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors.[Pubmed:14500756]
Mol Pharmacol. 2003 Oct;64(4):994-1005.
Lysophosphatidic acid (LPA) exerts a variety of biological responses through specific receptors: three subtypes of the EDG-family receptors, LPA1, LPA2, and LPA3 (formerly known as EDG-2, EDG-4, and EDG-7, respectively), and LPA4/GPR23, structurally distinct from the EDG-family receptors, have so far been identified. In the present study, we characterized the action mechanisms of 3-(4-[4-([1-(2-chlorophenyl)ethoxy]carbonyl amino)-3-methyl-5-isoxazolyl] benzylsulfanyl) propanoic acid (Ki16425) on the EDG-family LPA receptors. Ki16425 inhibited several responses specific to LPA, depending on the cell types, without any appreciable effect on the responses to other related lipid receptor agonists, including sphingosine 1-phosphate. With the cells overexpressing LPA1, LPA2, or LPA3, we examined the selectivity and mode of inhibition by Ki16425 against the LPA-induced actions and compared them with those of dioctyl glycerol pyrophosphate (DGPP 8:0), a recently identified antagonist for LPA receptors. Ki16425 inhibited the LPA-induced response in the decreasing order of LPA1 >/= LPA3 >> LPA2, whereas DGPP 8:0 preferentially inhibited the LPA3-induced actions. Ki16425 inhibited LPA-induced guanosine 5'-O-(3-thio)triphosphate binding as well as LPA receptor binding to membrane fractions with a same pharmacological specificity as in intact cells. The difference in the inhibition profile of Ki16425 and DGPP 8:0 was exploited for the evaluation of receptor subtypes involved in responses to LPA in A431 cells. Finally, Ki16425 also inhibited LPA-induced long-term responses, including DNA synthesis and cell migration. In conclusion, Ki16425 selectively inhibits LPA receptor-mediated actions, especially through LPA1 and LPA3; therefore, it may be useful in evaluating the role of LPA and its receptor subtypes involved in biological actions.


