YM-155 hydrochloridePotent survivin inhibitor CAS# 355406-09-6 |
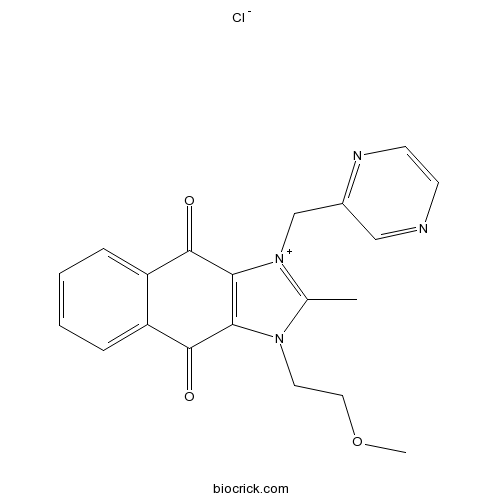
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 355406-09-6 | SDF | Download SDF |
| PubChem ID | 23108256 | Appearance | Powder |
| Formula | C20H19ClN4O3 | M.Wt | 398.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 55 mg/ml in DMSO and to 89 mg/ml in water | ||
| Chemical Name | 1-(2-methoxyethyl)-2-methyl-3-(pyrazin-2-ylmethyl)benzo[f]benzimidazol-3-ium-4,9-dione;chloride | ||
| SMILES | CC1=[N+](C2=C(N1CCOC)C(=O)C3=CC=CC=C3C2=O)CC4=NC=CN=C4.[Cl-] | ||
| Standard InChIKey | NUZWGSASIPTGSA-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C20H19N4O3.ClH/c1-13-23(9-10-27-2)17-18(24(13)12-14-11-21-7-8-22-14)20(26)16-6-4-3-5-15(16)19(17)25;/h3-8,11H,9-10,12H2,1-2H3;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | YM-155 hydrochloride is a potent and small-molecule inhibitor of survivin with IC50 value of 0.54 nM. | |||||
| Targets | survivin | |||||
| IC50 | 0.54 nM | |||||

YM-155 hydrochloride Dilution Calculator

YM-155 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5073 mL | 12.5364 mL | 25.0727 mL | 50.1454 mL | 62.6818 mL |
| 5 mM | 0.5015 mL | 2.5073 mL | 5.0145 mL | 10.0291 mL | 12.5364 mL |
| 10 mM | 0.2507 mL | 1.2536 mL | 2.5073 mL | 5.0145 mL | 6.2682 mL |
| 50 mM | 0.0501 mL | 0.2507 mL | 0.5015 mL | 1.0029 mL | 1.2536 mL |
| 100 mM | 0.0251 mL | 0.1254 mL | 0.2507 mL | 0.5015 mL | 0.6268 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
YM-155 hydrochloride is a novel small-molecule suppressant of surviving, the smallest member of inhibitor of apoptosis (IAP) gene family. It exhibits a potent suppressive activity against survivin but has little effect on expression levels of other IAP family members or B-cell lymphoma 2 (BCL-2) related proteins. YM155 also suppresses proliferation in a broad range of human cancer cell lines, induces tumor regression in non-small cell lung cancer (NSCLC), melanoma, bladder, aggressive non-Hodgkin lymphoma, and breast cancer xenograft models, reduces spontaneous metastases, and significantly prolongs the survival of animal harboring established metastatic tumors derived from a human triple-negative breast cancer (TNBC) cell lines.
References:
1.Naoki Kaneko, Kentaro Yamanaka, Aya Kita, Kenji Tabata, Takafumi Akabane, and Masamichi Mori. Synergistic antitumor activities of sepantronium bromide (YM155), a surviving suppressant, in combination with microtubule-targeting agents in triple-negative breast cancer cells. Biol Pharm Bull 2013.
2.Yan-Fang Tao, Jun Lu, Xiao-Juan Du, Li-Chao Sun, Xuan Zhao, Liang Peng, Lan Cao, Pei-Fang Xiao, Li Pang, Dong Wu, Na Wang, Xing Feng, Yan-Hong Li, Jian Ni, Jian Wang and Jian Pan. Survivin selective inhibitor YM155 induce apoptosis in SK-NEP-1 Wilms tumor cells. BMC Cancer 2012, 12:619
- 6'-O-p-Hydroxybenzoylcatalposide
Catalog No.:BCN5297
CAS No.:355143-38-3
- Ki16425
Catalog No.:BCC1155
CAS No.:355025-24-0
- Ki16198
Catalog No.:BCC4560
CAS No.:355025-13-7
- TCS 1205
Catalog No.:BCC7819
CAS No.:355022-97-8
- 3-beta-O-(trans-p-Coumaroyl)maslinic acid
Catalog No.:BCN1452
CAS No.:35482-91-8
- Balicatib
Catalog No.:BCC5139
CAS No.:354813-19-7
- SC-514
Catalog No.:BCC4554
CAS No.:354812-17-2
- Corianin
Catalog No.:BCN5296
CAS No.:35481-77-7
- Hirsuteine
Catalog No.:BCN2756
CAS No.:35467-43-7
- Caraphenol A
Catalog No.:BCN5295
CAS No.:354553-35-8
- INO-1001
Catalog No.:BCC2212
CAS No.:3544-24-9
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Buflomedil HCl
Catalog No.:BCC4760
CAS No.:35543-24-9
- N,N-dimethyl-2-Quinoxalinamine
Catalog No.:BCC9066
CAS No.:35552-76-2
- tert-Butyl rosuvastatin
Catalog No.:BCC9163
CAS No.:355806-00-7
- Betmidin
Catalog No.:BCN8253
CAS No.:35589-22-1
- Fluocinonide
Catalog No.:BCC4953
CAS No.:356-12-7
- Darapladib
Catalog No.:BCC1515
CAS No.:356057-34-6
- Toceranib
Catalog No.:BCC2005
CAS No.:356068-94-5
- N-Desethyl Sunitinib
Catalog No.:BCC1792
CAS No.:356068-97-8
- CP 20961
Catalog No.:BCC6063
CAS No.:35607-20-6
- Mesuaxanthone A
Catalog No.:BCN5298
CAS No.:3561-81-7
- Benzbromarone
Catalog No.:BCC4634
CAS No.:3562-84-3
- NSC 3852
Catalog No.:BCC2423
CAS No.:3565-26-2
Gateways to clinical trials.[Pubmed:18597009]
Methods Find Exp Clin Pharmacol. 2008 Apr;30(3):231-51.
(-)-Epigallocatechin gallate, (-)-Gossypol; Ad.hIFN-beta, AF-37702, Agatolimod sodium, Agomelatine, Alvocidib hydrochloride, ARC-1779; Belimumab, BIBW-2992, Binodenoson, Bortezomib, Bosutinib, Brivaracetam; Cediranib, Clevidipine, CNTO-328, CP-751871, Curcumin; Darapladib, Deforolimus, Denosumab, Desvenlafaxine succinate, Dipyridamole/prednisolone, Dronedarone hydrochloride, DTPw-HBV/Hib 2.5; Ecogramostim, Elacytarabine, Eltrombopag, Eprodisate sodium; Farnesylthiosalicylic acid, Febuxostat, Fenretinide, Ferumoxytol, FMP2.1/AS02A, Forodesine hydrochloride, FP-0011; HuLuc-63, Human Fibroblast Growth Factor 1; Idraparinux sodium, Indium 111 (111In) ibritumomab tiuxetan, Interleukin-21, Ipilimumab, ISS-1018, ITF-2357; Lapaquistat acetate, Laropiprant, Liposomal vincristine, LY-518674; Masitinib mesylate, MAXY-G34, MGCD-0103, Midostaurin, Mitumprotimut-T, MK-0343, MLN-1202, MM-093, Motexafin gadolinium; NB-001, NB-002, Niacin/laropiprant; Oblimersen sodium, Ocrelizumab, Omacetaxine mepesuccinate; Panobinostat, Patupilone, PBI-1402, Perifosine, PHA-739358, Plerixafor hydrochloride, Prasugrel; Regadenoson, RHAMM R3 peptide, Rilonacept, Rivaroxaban, Romiplostim; Safinamide mesilate, Salinosporamide A, Selenite sodium, Sotrastaurin; Thrombin alfa, Tipifarnib, TRO-19622; Vatalanib succinate, Vernakalant hydrochloride, VRC-WNVDNA017-00-VP; YM-155, Yttrium 90 (90Y) ibritumomab tiuxetan; Zosuquidar trihydrochloride.
Overcoming erlotinib resistance in EGFR mutation-positive non-small cell lung cancer cells by targeting survivin.[Pubmed:22075159]
Mol Cancer Ther. 2012 Jan;11(1):204-13.
Loss of PTEN was recently shown to contribute to resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) in EGFR mutation-positive non-small cell lung cancer (NSCLC) through activation of the protein kinase AKT. We previously showed that downregulation of the expression of the antiapoptotic protein survivin by EGFR-TKIs contributes to EGFR-TKI-induced apoptosis in EGFR mutation-positive NSCLC cells. We have now investigated the role of survivin expression in EGFR-TKI resistance induced by PTEN loss. The EGFR-TKI erlotinib did not affect survivin expression or induce apoptosis in EGFR mutation-positive NSCLC cells with PTEN loss. Downregulation of survivin either by transfection with a specific short interfering RNA or by exposure to the small-molecule survivin suppressor YM155 reversed erlotinib resistance in such cells in vitro. Furthermore, combination therapy with YM155 and erlotinib inhibited the growth of tumors formed by EGFR mutation-positive, PTEN-deficient NSCLC cells in nude mice to a greater extent than did treatment with either drug alone. These results thus indicate that persistent activation of signaling by the AKT-survivin pathway induced by PTEN loss underlies a mechanism of resistance to erlotinib-induced apoptosis in EGFR mutation-positive NSCLC. They further suggest that the targeting of survivin has the potential to overcome EGFR-TKI resistance in EGFR mutation-positive NSCLC.
Gateways to clinical trials.[Pubmed:18560631]
Methods Find Exp Clin Pharmacol. 2008 Mar;30(2):149-71.
Gateways to Clinical Trials are a guide to the most recent clinical trials in current literature and congresses. The data in the following tables has been retrieved from the Clinical Trials Knowledge Area of Prous Science Integrity, the drug discovery and development portal, http://integrity.prous.com. This issue focuses on the following selection of drugs: 131-I-Chlorotoxin, 423557; Abatacept, Ad.Egr.TNF.11D, Adalimumab, AE-941, Ambrisentan, AMR-001, Anacetrapib, Anakinra, Aripiprazole, Atazanavir sulfate; BAY-639044, Bazedoxifene acetate, Belimumab, Bevacizumab, Bortezomib, Botulinum toxin type B, Brivaracetam, Bucindolol hydrochloride; Carfilzomib, Carisbamate, CCX-282, CD20Bi, Ceftobiprole, Certolizumab pegol, CF-101, Cinacalcet hydrochloride, Cypher; Darifenacin hydrobromide, Degarelix acetate, Denosumab, Desvenlafaxine succinate, Dexlansoprazole, Dexverapamil, Drotrecogin alfa (activated), Duloxetine hydrochloride, Dutasteride; Efalizumab, EPs-7630, Escitalopram oxalate, Etoricoxib; Fluticasone furoate, Fondaparinux sodium, Fospropofol disodium; Hexadecyloxypropyl-cidofovir, HIV gp120/NefTat/AS02A, HPV-6/11/16/18; INCB-18424, Incyclinide, Inhalable human insulin, Insulin detemir; KNS-760704, KW-0761; Lacosamide, Lenalidomide, Levetiracetam, Licofelone, Lidocaine/prilocaine; mAb 216, MEDI-528, Men ACWY, Meningococcal C-CRM197 vaccine, Methylnaltrexone bromide; Nemifitide ditriflutate, Nicotine conjugate vaccine, Nilotinib hydrochloride monohydrate; Octaparin; Parathyroid hormone (human recombinant), Pegaptanib octasodium, Pitrakinra, Prasterone, Pregabalin; Ranelic acid distrontium salt, Rasagiline mesilate, Retigabine, Rimonabant, RTS,S/AS02D; Sarcosine, Sitaxentan sodium, Solifenacin succinate, Sunitinib malate; Taranabant, Taxus, Teduglutide, Teriparatide, Ticagrelor, Travoprost, TRU-015; USlipristal acetate, Urocortin 2; Vardenafil hydrochloride hydrate; YM-155, Yttrium 90 (90Y) ibritumomab tiuxetan; Zanolimumab, Zoledronic acid monohydrate, Zotarolimus, Zotarolimus-eluting stent.
Targeting the proliferative and chemoresistant compartment in chronic lymphocytic leukemia by inhibiting survivin protein.[Pubmed:24618734]
Leukemia. 2014 Oct;28(10):1993-2004.
Chronic lymphocytic leukemia (CLL) cells located in proliferation centers are constantly stimulated by accessory cells, which provide them with survival and proliferative signals and mediate chemotherapy resistance. Herein, we designed an experimental strategy with the aim of mimicking the microenvironment found in the proliferative centers to specifically target actively proliferating CLL cells. For this, we co-cultured CLL cells and bone marrow stromal cells with concomitant CD40 and Toll-like receptor 9 stimulation. This co-culture system induced proliferation, cell-cycle entry and marked resistance to treatment with fludarabine and bendamustine. Proliferating CLL cells clustered together showed a typical morphology of activated B cells and expressed survivin protein, a member of the inhibitor of apoptosis family that is mainly expressed by CLL cells in the proliferation centers. With the aim of specifically targeting actively proliferating and chemoresistant CLL cells, we investigated the effects of treatment with YM155, a small-molecule survivin inhibitor. YM155 treatment suppressed the co-culture-induced survivin expression and that was sufficient to inhibit proliferation and effectively induce apoptosis particularly in the proliferative subset of CLL cells. Interestingly, sensitivity to YM155 was independent from common prognostic markers, including 17p13.1 deletion. Altogether, these findings provide a rationale for clinical development of YM155 in CLL.
Combination of YM155, a survivin suppressant, with bendamustine and rituximab: a new combination therapy to treat relapsed/refractory diffuse large B-cell lymphoma.[Pubmed:24486595]
Clin Cancer Res. 2014 Apr 1;20(7):1814-22.
PURPOSE: There remains an unmet therapeutic need for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL). The purpose of this study was to evaluate the therapeutic potential of sepantronium bromide (YM155), a survivin suppressant, in combination with either bendamustine or both bendamustine and rituximab using DLBCL models. EXPERIMENTAL DESIGN: Human DLBCL cell lines, DB, SU-DHL-8, and WSU-DLCL2, were treated with YM155 in combination with bendamustine. Cell viability, apoptosis induction, protein expression, and cell-cycle distribution were evaluated. Furthermore, antitumor activities of YM155, in combination with bendamustine or both bendamustine and rituximab, were evaluated in mice bearing human DLBCL xenografts. RESULTS: The combination of YM155 with bendamustine showed greater cell growth inhibition and sub-G1 population than either agent alone. YM155 inhibited bendamustine-induced activation of the ATM pathway and accumulation of survivin at G2-M phase, with greater DNA damage and apoptosis than either single agent alone. In a DLBCL DB murine xenograft model, YM155 enhanced the antitumor activity of bendamustine, resulting in complete tumor regression without affecting body weight. Furthermore, YM155 combined with bendamustine and rituximab, decreased FLT-PET signals in lymph nodes and prolonged overall survival of mice bearing disseminated SU-DHL-8, an activated B-cell-like (ABC)-DLBCL xenografts when compared with the combination of either rituximab and bendamustine or YM155 with rituximab. CONCLUSIONS: These results support a clinical trial of the combination of YM155 with bendamustine and rituximab in relapsed/refractory DLBCL.


