Elvitegravir (GS-9137)HIV-1 integrase inhibitor,potent CAS# 697761-98-1 |
- GSK744 (S/GSK1265744)
Catalog No.:BCC3888
CAS No.:1051375-10-0
- S/GSK1349572
Catalog No.:BCC2138
CAS No.:1051375-16-6
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Raltegravir (MK-0518)
Catalog No.:BCC2137
CAS No.:518048-05-0
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
- MK-2048
Catalog No.:BCC2136
CAS No.:869901-69-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 697761-98-1 | SDF | Download SDF |
| PubChem ID | 23083982 | Appearance | Powder |
| Formula | C23H23ClFNO5 | M.Wt | 447.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GS-9137; JTK-303; EVG; D06677 | ||
| Solubility | DMSO : ≥ 50 mg/mL (111.64 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
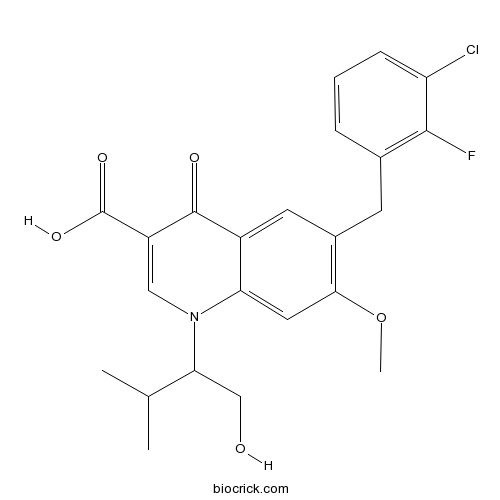
| Chemical Name | 6-[(3-chloro-2-fluorophenyl)methyl]-1-(1-hydroxy-3-methylbutan-2-yl)-7-methoxy-4-oxoquinoline-3-carboxylic acid | ||
| SMILES | CC(C)C(CO)N1C=C(C(=O)C2=CC(=C(C=C21)OC)CC3=C(C(=CC=C3)Cl)F)C(=O)O | ||
| Standard InChIKey | JUZYLCPPVHEVSV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H23ClFNO5/c1-12(2)19(11-27)26-10-16(23(29)30)22(28)15-8-14(20(31-3)9-18(15)26)7-13-5-4-6-17(24)21(13)25/h4-6,8-10,12,19,27H,7,11H2,1-3H3,(H,29,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Elvitegravir is a potent inhibitor of HIV-I integrase with IC50 value of 7.2 nM. | |||||
| Targets | HIV-I integrase | |||||
| IC50 | 7.2 nM | |||||

Elvitegravir (GS-9137) Dilution Calculator

Elvitegravir (GS-9137) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2326 mL | 11.1632 mL | 22.3264 mL | 44.6528 mL | 55.816 mL |
| 5 mM | 0.4465 mL | 2.2326 mL | 4.4653 mL | 8.9306 mL | 11.1632 mL |
| 10 mM | 0.2233 mL | 1.1163 mL | 2.2326 mL | 4.4653 mL | 5.5816 mL |
| 50 mM | 0.0447 mL | 0.2233 mL | 0.4465 mL | 0.8931 mL | 1.1163 mL |
| 100 mM | 0.0223 mL | 0.1116 mL | 0.2233 mL | 0.4465 mL | 0.5582 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Elvitegravir (also known as GS-9137 or JTK-303), a monoketo acid modified from the distinct diketo acid moiety (DKA) motif, is a potent inhibitor of human immunodeficiency virus type I (HIV-1) integrase, an enzyme integrating the viral DNA into the cellular DNA of the host during HIV replication. Elvitegravir inhibits the function of HIV-1 integrase through blocking viral DNA strand transfer and integration, which prevents the replication of HIV and subsequently allows viral DNA to be metabolized by cellular enzymes. Multiple studies indicate that elvitegravir not only inhibits the replication of a variety of subtypes of HIV-1 but also exhibits antiviral activity against murine leukemia virus (MLV) and simian immunodeficirncy virus (SIV).
Reference
Janice Soo Fern Lee, Alexandra Calmy, Isabelle Andrieux-Meyer, and Nathan Ford. Review of the safty, efficacy, and pharmacokinetics of elviteravir with an emphasis on resource-limited settings. HIV/AIDS- Research and Palliative Care 2012; 4: 5-15
Peter K Quashie, Richard D Sloan and Mark A Wainberg. Novel therapeutic strategies targeting HIV integrase. BMC Medicine 2012; 10:34
Kazuya Shimura, Eiichi Kodama, Yasuko Sakagami, Yuji Matsuzaki, Wataru Watanabe, Kazunobu Yamataka, Yasuo Watanabe, Yoshitsugu Ohata, Satoki Doi, Motohide Sato, Mitsuki Kano, Satoru Ikeda, and Masao Matsuoka. Broad antiretroviral activity and resistance profile of the novel human innunodeficiency virus integrase inhibitor elvitegravir. (JTK-303/GS-9137). Journal of Virology 2008; 764-774.
- Antibiotic BU 2313A
Catalog No.:BCN1846
CAS No.:69774-86-3
- 4-(3,4-Dimethoxyphenyl)-3-buten-1-ol
Catalog No.:BCN4258
CAS No.:69768-97-4
- W-9 hydrochloride
Catalog No.:BCC6623
CAS No.:69762-85-2
- BIS-TRIS
Catalog No.:BCC8028
CAS No.:6976-37-0
- CRSP-1
Catalog No.:BCC6043
CAS No.:697327-12-1
- Silvestrol
Catalog No.:BCC1948
CAS No.:697235-38-4
- 6-Amino-1-methyl-5-nitrosouracil
Catalog No.:BCC8756
CAS No.:6972-78-7
- DCB
Catalog No.:BCC7212
CAS No.:6971-97-7
- VIP (6-28) (human, rat, porcine, bovine)
Catalog No.:BCC5838
CAS No.:69698-54-0
- 2,5-Dimethylchroman-4-one
Catalog No.:BCN7200
CAS No.:69687-87-2
- Oleaside A
Catalog No.:BCN6772
CAS No.:69686-84-6
- Mepiroxol
Catalog No.:BCC3810
CAS No.:6968-72-5
- UAMC 00039 dihydrochloride
Catalog No.:BCC6340
CAS No.:697797-51-6
- Swertiajaponin
Catalog No.:BCN2791
CAS No.:6980-25-2
- 2,7-Dihydroxy-2H-1,4-benzoxazin-3(4H)-one
Catalog No.:BCN1374
CAS No.:69804-59-7
- Agarotetrol
Catalog No.:BCN6763
CAS No.:69809-22-9
- Noradrenaline Bitartrate
Catalog No.:BCC8343
CAS No.:51-40-1
- Neratinib (HKI-272)
Catalog No.:BCC3685
CAS No.:698387-09-6
- Bourjotinolone A
Catalog No.:BCN4259
CAS No.:6985-35-9
- Boc-Glu(OtBu)-ONp
Catalog No.:BCC3393
CAS No.:69876-58-0
- Petunidin-3-O-glucoside chloride
Catalog No.:BCN3025
CAS No.:6988-81-4
- Pseudoginsenoside F11
Catalog No.:BCN1062
CAS No.:69884-00-0
- Atractylone
Catalog No.:BCN3048
CAS No.:6989-21-5
- Bayogenin
Catalog No.:BCN2458
CAS No.:6989-24-8
Broad antiretroviral activity and resistance profile of the novel human immunodeficiency virus integrase inhibitor elvitegravir (JTK-303/GS-9137).[Pubmed:17977962]
J Virol. 2008 Jan;82(2):764-74.
Integrase (IN), an essential enzyme of human immunodeficiency virus (HIV), is an attractive antiretroviral drug target. The antiviral activity and resistance profile in vitro of a novel IN inhibitor, elvitegravir (EVG) (also known as JTK-303/GS-9137), currently being developed for the treatment of HIV-1 infection are described. EVG blocked the integration of HIV-1 cDNA through the inhibition of DNA strand transfer. EVG inhibited the replication of HIV-1, including various subtypes and multiple-drug-resistant clinical isolates, and HIV-2 strains with a 50% effective concentration in the subnanomolar to nanomolar range. EVG-resistant variants were selected in two independent inductions, and a total of 8 amino acid substitutions in the catalytic core domain of IN were observed. Among the observed IN mutations, T66I and E92Q substitutions mainly contributed to EVG resistance. These two primary resistance mutations are located in the active site, and other secondary mutations identified are proximal to these primary mutations. The EVG-selected IN mutations, some of which represent novel IN inhibitor resistance mutations, conferred reduced susceptibility to other IN inhibitors, suggesting that a common mechanism is involved in resistance and potential cross-resistance. The replication capacity of EVG-resistant variants was significantly reduced relative to both wild-type virus and other IN inhibitor-resistant variants selected by L-870,810. EVG and L-870,810 both inhibited the replication of murine leukemia virus and simian immunodeficiency virus, suggesting that IN inhibitors bind to a conformationally conserved region of various retroviral IN enzymes and are an ideal drug for a range of retroviral infections.


