BIS-TRISCAS# 6976-37-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6976-37-0 | SDF | Download SDF |
| PubChem ID | 81462 | Appearance | Powder |
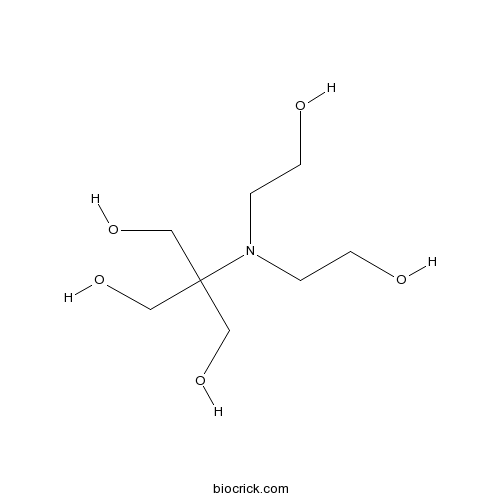
| Formula | C8H19NO5 | M.Wt | 209.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2000 mM in water | ||
| Chemical Name | 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol | ||
| SMILES | C(CO)N(CCO)C(CO)(CO)CO | ||
| Standard InChIKey | OWMVSZAMULFTJU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H19NO5/c10-3-1-9(2-4-11)8(5-12,6-13)7-14/h10-14H,1-7H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Commonly used buffering agent. |

BIS-TRIS Dilution Calculator

BIS-TRIS Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7792 mL | 23.896 mL | 47.792 mL | 95.584 mL | 119.48 mL |
| 5 mM | 0.9558 mL | 4.7792 mL | 9.5584 mL | 19.1168 mL | 23.896 mL |
| 10 mM | 0.4779 mL | 2.3896 mL | 4.7792 mL | 9.5584 mL | 11.948 mL |
| 50 mM | 0.0956 mL | 0.4779 mL | 0.9558 mL | 1.9117 mL | 2.3896 mL |
| 100 mM | 0.0478 mL | 0.239 mL | 0.4779 mL | 0.9558 mL | 1.1948 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CRSP-1
Catalog No.:BCC6043
CAS No.:697327-12-1
- Silvestrol
Catalog No.:BCC1948
CAS No.:697235-38-4
- 6-Amino-1-methyl-5-nitrosouracil
Catalog No.:BCC8756
CAS No.:6972-78-7
- DCB
Catalog No.:BCC7212
CAS No.:6971-97-7
- VIP (6-28) (human, rat, porcine, bovine)
Catalog No.:BCC5838
CAS No.:69698-54-0
- 2,5-Dimethylchroman-4-one
Catalog No.:BCN7200
CAS No.:69687-87-2
- Oleaside A
Catalog No.:BCN6772
CAS No.:69686-84-6
- Mepiroxol
Catalog No.:BCC3810
CAS No.:6968-72-5
- (Z-Cys-OH)2
Catalog No.:BCC2917
CAS No.:6968-11-2
- 6-Aminoindazole
Catalog No.:BCC8762
CAS No.:6967-12-0
- Tanshinone IIA-sulfonic sodium
Catalog No.:BCN2541
CAS No.:69659-80-9
- Didanosine
Catalog No.:BCC3763
CAS No.:69655-05-6
- W-9 hydrochloride
Catalog No.:BCC6623
CAS No.:69762-85-2
- 4-(3,4-Dimethoxyphenyl)-3-buten-1-ol
Catalog No.:BCN4258
CAS No.:69768-97-4
- Antibiotic BU 2313A
Catalog No.:BCN1846
CAS No.:69774-86-3
- Elvitegravir (GS-9137)
Catalog No.:BCC2134
CAS No.:697761-98-1
- UAMC 00039 dihydrochloride
Catalog No.:BCC6340
CAS No.:697797-51-6
- Swertiajaponin
Catalog No.:BCN2791
CAS No.:6980-25-2
- 2,7-Dihydroxy-2H-1,4-benzoxazin-3(4H)-one
Catalog No.:BCN1374
CAS No.:69804-59-7
- Agarotetrol
Catalog No.:BCN6763
CAS No.:69809-22-9
- Noradrenaline Bitartrate
Catalog No.:BCC8343
CAS No.:51-40-1
- Neratinib (HKI-272)
Catalog No.:BCC3685
CAS No.:698387-09-6
- Bourjotinolone A
Catalog No.:BCN4259
CAS No.:6985-35-9
- Boc-Glu(OtBu)-ONp
Catalog No.:BCC3393
CAS No.:69876-58-0
Crystal structure of bis-[tris-(1,10-phenanthroline-kappa(2) N,N')cobalt(II)] tetra-nitrate N,N'-(1,4-phenyl-enedicarbon-yl)diglycine solvate octa-hydrate.[Pubmed:26396753]
Acta Crystallogr E Crystallogr Commun. 2015 Jul 11;71(Pt 8):910-4.
The complex cation of the title compound, [Co(C12H8N2)3]2(NO3)4.C12H12N2O6.8H2O, contains a Co(II) atom with a distorted octa-hedral coordination environment defined by six N atoms from three bidentate 1,10-phenanthroline ligands. The asymmetric unit of the title compound is completed by one-half of the N,N'-(1,4-phenyl-enedicarbon-yl)diglycine solvent mol-ecule, which is located on a centre of inversion, by two nitrate counter-anions and four solvent water mol-ecules. Two [Co(C12H8N2)3](2+) cations are connected through C-Hcdots, three dots, centeredO contacts and through lone-paircdots, three dots, centeredpi inter-actions involving the non-coordinating N,N'-(1,4-phenyl-enedicarbon-yl)diglycine and phenanthroline mol-ecules. The different aromatic ring systems are involved in pi-pi stacking and C-Hcdots, three dots, centeredpi inter-actions, with centroid-to-centroid distances in the range 3.7094 (8)-3.9973 (9) A. The crystal structure is stabilized by further anioncdots, three dots, centeredpi inter-actions and C-Hcdots, three dots, centeredO contacts, as well as O-Hcdots, three dots, centeredO and N-Hcdots, three dots, centeredO hydrogen bonds between water mol-ecules, the non-coordinating nitrate anions, N,N'-(1,4-phenyl-enedicarbon-yl)diglycine and phenanthroline mol-ecules. These non-covalent inter-actions give rise to a three-dimensional supra-molecular network.
Construction of bis-, tris- and tetrahydrazones by addition of azoalkenes to amines and ammonia.[Pubmed:28144315]
Beilstein J Org Chem. 2016 Nov 21;12:2471-2477.
Exhaustive Michael-type alkylations of amines and ammonia with azoalkenes (generated from alpha-halohydrazones) were demonstrated as an efficient approach to poly(hydrazonomethyl)amines - a novel class of polynitrogen ligands. An intramolecular cyclotrimerization of C=N bonds in tris(hydrazonomethyl)amine to the respective 1,4,6,10-tetraazaadamantane derivative was demonstrated.
Crystal structure of bis-(eta(5)-cyclo-penta-dien-yl)(1,4-di-tert-butyl-buta-1-en-3-yn-1-yl)zirconium( IV) mu2-hydroxido-bis-[tris(penta-fluoro-phen-yl)borate].[Pubmed:25844214]
Acta Crystallogr E Crystallogr Commun. 2015 Feb 28;71(Pt 3):m71-2.
Alkyl zirconocene cations have been of considerable inter-est as reactive species in many polymerization processes. In the crystal structure of the title compound, [Zr(C12H19)(C5H5)2](C36HB2F30O), the [Zr(C5H5)2((t-Bu)C=C(H)-C2(t-Bu))](+) cation displays a buta-1-en-3-yne ligand side-on coordinated to a typical bent zirconocene [centroid(cp)-Zr-centroid(cp) = 131.4 (3) degrees , Zr-C(buta-1-en-3-yne) = 2.255 (3), 2.597 (3) and 2.452 (2) A]. In the [HO(B(C6F5)3)2](-) anion, intra-molecular O-Hcdots, three dots, centeredF hydrogen bonds are observed. One tert-butyl group in the complex cation is disordered over two sets of sites with occupancies 0.701(4):0.299(4).
Crystal structure of trans-dihydrido-bis[tris-(di-methyl-amino)-phosphane-kappaP]platinum(II).[Pubmed:26029413]
Acta Crystallogr E Crystallogr Commun. 2015 Mar 14;71(Pt 4):m83-4.
The mol-ecule of the title compound, [PtH2(C6H18N3P)2], has a centrosymmetric square-planar structure in which the Pt(II) atom is bonded to two H and two P atoms in a mutually trans configuration. The Pt(II) atom sits on an inversion center and thus the asymmetric unit contains only half the mol-ecule. The Pt-P and Pt-H distances are 2.2574 (10) and 1.49 (7) A, respectively.


