UAMC 00039 dihydrochlorideDipeptidyl peptidase II (DPP-II) inhibitor CAS# 697797-51-6 |
- GDC-0623
Catalog No.:BCC4150
CAS No.:1168091-68-6
- Pimasertib (AS-703026)
Catalog No.:BCC2529
CAS No.:1236699-92-5
- PD184352 (CI-1040)
Catalog No.:BCC1112
CAS No.:212631-79-3
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- PD318088
Catalog No.:BCC2539
CAS No.:391210-00-7
- RO4987655
Catalog No.:BCC5135
CAS No.:874101-00-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 697797-51-6 | SDF | Download SDF |
| PubChem ID | 24757888 | Appearance | Powder |
| Formula | C16H26Cl3N3O | M.Wt | 382.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 150 mg/mL (391.89 mM) *"≥" means soluble, but saturation unknown. | ||
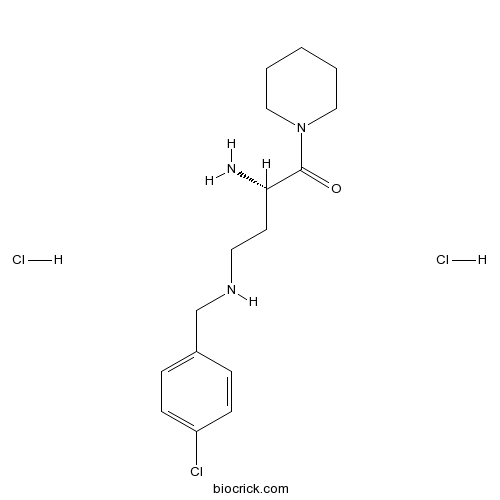
| Chemical Name | (2S)-2-amino-4-[(4-chlorophenyl)methylamino]-1-piperidin-1-ylbutan-1-one;dihydrochloride | ||
| SMILES | C1CCN(CC1)C(=O)C(CCNCC2=CC=C(C=C2)Cl)N.Cl.Cl | ||
| Standard InChIKey | IWXMOQGMIWZNPR-CKUXDGONSA-N | ||
| Standard InChI | InChI=1S/C16H24ClN3O.2ClH/c17-14-6-4-13(5-7-14)12-19-9-8-15(18)16(21)20-10-2-1-3-11-20;;/h4-7,15,19H,1-3,8-12,18H2;2*1H/t15-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of dipeptidyl peptidase II (DPP-II) (IC50 = 0.48 nM). Exhibits selectivity for DPP-II against DPP-9, DPP-8 and DPP-IV (IC50 values are 78.6, 142 and 165 μM, respectively). Orally available. |

UAMC 00039 dihydrochloride Dilution Calculator

UAMC 00039 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6126 mL | 13.063 mL | 26.126 mL | 52.2521 mL | 65.3151 mL |
| 5 mM | 0.5225 mL | 2.6126 mL | 5.2252 mL | 10.4504 mL | 13.063 mL |
| 10 mM | 0.2613 mL | 1.3063 mL | 2.6126 mL | 5.2252 mL | 6.5315 mL |
| 50 mM | 0.0523 mL | 0.2613 mL | 0.5225 mL | 1.045 mL | 1.3063 mL |
| 100 mM | 0.0261 mL | 0.1306 mL | 0.2613 mL | 0.5225 mL | 0.6532 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
UAMC00039 dihydrochloride is a potent, reversible and competitive dipeptidyl peptidase II inhibitor with an IC50 of 0.48 nM.
In Vitro:UAMC00039 has an IC50 of 0.48±0.04 nM and a high selectivity for DPPII compared to DPPIV (IC50=165±9 µM) and DPP activity not caused by DPPII or DPPIV. UAMC00039 seems a promising tool to unravel the function of DPPII as well as to validate its potential as a therapeutic target[1]. The efficacy of a DPPII inhibitor in cell culture depends not only on the inhibitors’ potency towards the enzyme but also on its stability in the medium and its ability to enter the cell. UAMC00039 is stable for at least 48 h at 37 °C in culture medium and in DPPII assay buffer. The compound is able to enter PBMC within 1 min resulting in a concentration-dependent inhibition of intracellular DPPII activity without affecting the ‘non-DPPII’ DPP activity. 1 and 100 μM UAMC00039 inhibits DPPII activity of PBMC and U937 cells more than 90%[2].
In Vivo:A dose dependent inhibition of DPPII but not of DPPIV is observed in the peripheral organs of both the rats and the mice (after oral administration) and the rabbits (after IV administration). UAMC00039 tested orally at 2 mg/kg does not cause signs of acute toxicity and does not cause any significant changes in the following functions that are evaluated: general behaviour, body temperature, respiration, bleeding time, blood pressure, urine volume, liver function, fasting glucose and gastrointestinal parameters like acidity, motility and irritation[1].
References:
[1]. Maes MB, et al. In vivo effects of a potent, selective DPPII inhibitor: UAMC00039 is a possible tool for the elucidation of the physiological function of DPPII. Adv Exp Med Biol. 2006;575:73-85.
[2]. Maes MB, et al. Dipeptidyl peptidase II and leukocyte cell death. Biochem Pharmacol. 2006 Jun 28;72(1):70-9.
- Elvitegravir (GS-9137)
Catalog No.:BCC2134
CAS No.:697761-98-1
- Antibiotic BU 2313A
Catalog No.:BCN1846
CAS No.:69774-86-3
- 4-(3,4-Dimethoxyphenyl)-3-buten-1-ol
Catalog No.:BCN4258
CAS No.:69768-97-4
- W-9 hydrochloride
Catalog No.:BCC6623
CAS No.:69762-85-2
- BIS-TRIS
Catalog No.:BCC8028
CAS No.:6976-37-0
- CRSP-1
Catalog No.:BCC6043
CAS No.:697327-12-1
- Silvestrol
Catalog No.:BCC1948
CAS No.:697235-38-4
- 6-Amino-1-methyl-5-nitrosouracil
Catalog No.:BCC8756
CAS No.:6972-78-7
- DCB
Catalog No.:BCC7212
CAS No.:6971-97-7
- VIP (6-28) (human, rat, porcine, bovine)
Catalog No.:BCC5838
CAS No.:69698-54-0
- 2,5-Dimethylchroman-4-one
Catalog No.:BCN7200
CAS No.:69687-87-2
- Oleaside A
Catalog No.:BCN6772
CAS No.:69686-84-6
- Swertiajaponin
Catalog No.:BCN2791
CAS No.:6980-25-2
- 2,7-Dihydroxy-2H-1,4-benzoxazin-3(4H)-one
Catalog No.:BCN1374
CAS No.:69804-59-7
- Agarotetrol
Catalog No.:BCN6763
CAS No.:69809-22-9
- Noradrenaline Bitartrate
Catalog No.:BCC8343
CAS No.:51-40-1
- Neratinib (HKI-272)
Catalog No.:BCC3685
CAS No.:698387-09-6
- Bourjotinolone A
Catalog No.:BCN4259
CAS No.:6985-35-9
- Boc-Glu(OtBu)-ONp
Catalog No.:BCC3393
CAS No.:69876-58-0
- Petunidin-3-O-glucoside chloride
Catalog No.:BCN3025
CAS No.:6988-81-4
- Pseudoginsenoside F11
Catalog No.:BCN1062
CAS No.:69884-00-0
- Atractylone
Catalog No.:BCN3048
CAS No.:6989-21-5
- Bayogenin
Catalog No.:BCN2458
CAS No.:6989-24-8
- Evodine
Catalog No.:BCN2630
CAS No.:6989-38-4
Structure-activity relationship studies on isoindoline inhibitors of dipeptidyl peptidases 8 and 9 (DPP8, DPP9): is DPP8-selectivity an attainable goal?[Pubmed:21711053]
J Med Chem. 2011 Aug 25;54(16):5737-46.
This work represents the first directed study to identify modification points in the topology of a representative DPP8/9-inhibitor, capable of rendering selectivity for DPP8 over DPP9. The availability of a DPP8-selective compound would be highly instrumental for studying and untwining the biological roles of DPP8 and DPP9 and for the disambiguation of biological effects of nonselective DPP-inhibitors that have mainly been ascribed to blocking of DPPIV's action. The cell-permeable DPP8/9-inhibitor 7 was selected as a lead and dissected into several substructures that were modified separately for evaluating their potential to contribute to selectivity. The obtained results, together with earlier work from our group, clearly narrow down the most probable DPP8-selectivity imparting modification points in DPP8/9 inhibitors to parts of space that are topologically equivalent to the piperazine ring system in 7. This information can be considered of high value for future design of compounds with maximal DPP8 selectivity.
Dipeptidyl peptidase 8/9-like activity in human leukocytes.[Pubmed:17287297]
J Leukoc Biol. 2007 May;81(5):1252-7.
The proline-specific dipeptidyl peptidases (DPPs) are emerging as a protease family with important roles in the regulation of signaling by peptide hormones. Inhibitors of DPPs have an intriguing, therapeutic potential, with clinical efficacy seen in patients with diabetes. Until now, only recombinant forms of DPP8 and DPP9 have been characterized. Their enzymatic activities have not been demonstrated in or purified from any natural source. Using several selective DPP inhibitors, we show that DPP activity, attributable to DPP8/9 is present in human PBMC. All leukocyte types tested (lymphocytes, monocytes, Jurkat, and U937 cells) were shown to contain similar DPP8/9-specific activities, and DPPII- and DPPIV-specific activities varied considerably. The results were confirmed by DPPIV/CD26 immunocapture experiments. Subcellular fractionation localized the preponderance of DPP8/9 activity to the cytosol and DPPIV in the membrane fractions. Using Jurkat cell cytosol as a source, a 30-fold, enriched DPP preparation was obtained, which had enzymatic characteristics closely related to the ones of DPP8 and/or -9, including inhibition by allo-Ile-isoindoline and affinity for immobilized Lys-isoindoline.


