PD318088Allosteric MEK1/2 inhibitor, non-ATP competitive CAS# 391210-00-7 |
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- PD98059
Catalog No.:BCC1098
CAS No.:167869-21-8
- PD184352 (CI-1040)
Catalog No.:BCC1112
CAS No.:212631-79-3
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 391210-00-7 | SDF | Download SDF |
| PubChem ID | 10231331 | Appearance | Powder |
| Formula | C16H13BrF3IN2O4 | M.Wt | 561.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (178.22 mM) *"≥" means soluble, but saturation unknown. | ||
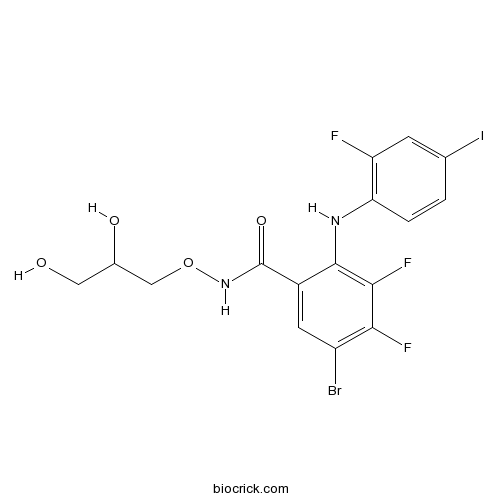
| Chemical Name | 5-bromo-N-(2,3-dihydroxypropoxy)-3,4-difluoro-2-(2-fluoro-4-iodoanilino)benzamide | ||
| SMILES | C1=CC(=C(C=C1I)F)NC2=C(C(=C(C=C2C(=O)NOCC(CO)O)Br)F)F | ||
| Standard InChIKey | XXSSGBYXSKOLAM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H13BrF3IN2O4/c17-10-4-9(16(26)23-27-6-8(25)5-24)15(14(20)13(10)19)22-12-2-1-7(21)3-11(12)18/h1-4,8,22,24-25H,5-6H2,(H,23,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PD318088 is a non-ATP competitive allosteric MEK1/2 inhibitor | |||||
| Targets | MEK1/2 | |||||

PD318088 Dilution Calculator

PD318088 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7822 mL | 8.9112 mL | 17.8225 mL | 35.6449 mL | 44.5561 mL |
| 5 mM | 0.3564 mL | 1.7822 mL | 3.5645 mL | 7.129 mL | 8.9112 mL |
| 10 mM | 0.1782 mL | 0.8911 mL | 1.7822 mL | 3.5645 mL | 4.4556 mL |
| 50 mM | 0.0356 mL | 0.1782 mL | 0.3564 mL | 0.7129 mL | 0.8911 mL |
| 100 mM | 0.0178 mL | 0.0891 mL | 0.1782 mL | 0.3564 mL | 0.4456 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PD318088 is an allosteric and non-ATP competitive MEK1/2 inhibitor.
PD318088 is an analog of PD184352, which means it might have important anti-proliferative activity against cancer cells, although no functional study of PD318088 is currently available. PD318088 binds simultaneously with ATP in a region of the MEK1 active site that is adjacent to the ATP-binding site. Formation of the ternary complexes with PD318088 and MgATP results in moderate increases (to 140 nM) for the Kd monomer-dimer for both MEK1 and MEK2. PD318088 and MgATP together increase the dimerization disassociation constant for MEK1 and MEK2 slightly from ~75 nM to ~140 nM [1].
PD318088 is a biarylamine compound co-crystallized with MEK. G8935 occupies the unique pocket adjacent to ATP binding site and is uperimposed with PD318088 .PD318088 averts to use the pocket formed by Ile216, Phe209, Arg189, and Asp190. [2]
References:
1. Ohren JF1, Chen H, Pavlovsky A et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat Struct Mol Biol. 2004 Dec;11(12):1192-7. Epub 2004 Nov 14.
2. Han S, Zhou V, Pan S et al. Identification of coumarin derivatives as a novel class of allosteric MEK1 inhibitors. Bioorg Med Chem Lett. 2005 Dec 15;15(24):5467-73. Epub 2005 Sep 30.
- Nigranoic acid
Catalog No.:BCN2399
CAS No.:39111-07-4
- Ajmalan-17-one
Catalog No.:BCN3519
CAS No.:3911-19-1
- Karakoline
Catalog No.:BCC8331
CAS No.:39089-30-0
- UCM 707
Catalog No.:BCC7217
CAS No.:390824-20-1
- Ingenol 20-palmitate
Catalog No.:BCN7678
CAS No.:39071-33-5
- GS 39783
Catalog No.:BCC7233
CAS No.:39069-52-8
- Buxbodine D
Catalog No.:BCN5448
CAS No.:390362-53-5
- Buxbodine B
Catalog No.:BCN5447
CAS No.:390362-51-3
- 9-Methoxycamptothecine
Catalog No.:BCN1219
CAS No.:39026-92-1
- Guggulsterone E
Catalog No.:BCC8181
CAS No.:39025-24-6
- Z-Guggulsterone
Catalog No.:BCC7712
CAS No.:39025-23-5
- 3-Epiwilsonine
Catalog No.:BCN5446
CAS No.:39024-15-2
- PD0325901
Catalog No.:BCC1277
CAS No.:391210-10-9
- N-Me-Ala-OH.HCl
Catalog No.:BCC2619
CAS No.:3913-67-5
- Trimebutine
Catalog No.:BCC4615
CAS No.:39133-31-8
- Ac-His-OH.H2O
Catalog No.:BCC2953
CAS No.:39145-52-3
- (E)-Cinnamyl-(Z)-p-coumarate
Catalog No.:BCN7694
CAS No.:391682-51-2
- Oxyresveratrol 2-O-beta-D-glucopyranoside
Catalog No.:BCN1448
CAS No.:392274-22-5
- Ginsenoside Compound K
Catalog No.:BCN1246
CAS No.:39262-14-1
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Tiplaxtinin(PAI-039)
Catalog No.:BCC6439
CAS No.:393105-53-8
- 2,3-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN6534
CAS No.:3934-81-4
- BCTC
Catalog No.:BCC7797
CAS No.:393514-24-4
- Neurotensin
Catalog No.:BCC5842
CAS No.:39379-15-2
Identification of coumarin derivatives as a novel class of allosteric MEK1 inhibitors.[Pubmed:16199156]
Bioorg Med Chem Lett. 2005 Dec 15;15(24):5467-73.
A homogenous TR-FRET-based in vitro coupling assay for the MAP3Ks-MEK1-ERK2 kinase cascade was established and was used to screen for inhibitors of the ERK/MAPK pathway. A series of coumarin derivatives were identified from the screen. These compounds potently inhibit the activation of the unactivated human MEK1 by upstream MAP3Ks (including BRAF and COT), but do not inhibit the activity of the activated MEK1. In addition, the potency of these compounds in inhibiting MEK1 activation is not affected by varying the ATP concentration, suggesting that these inhibitors are not competitive with ATP. As expected, the coumarin compounds potently inhibit LPS-induced TNFalpha production and ERK phosphorylation in THP-1 cells, with the most potent compound having an IC(50) of 90nM. Molecular modeling studies suggest that these coumarins bind to an allosteric site in the inactive conformation of MEK1. This site has been shown to be utilized by the biarylamine series of MEK inhibitors such as PD318088. Very interestingly, the identified coumarin derivatives are almost identical to a series of inhibitors recently reported that block LPS-induced TNFalpha production. Our findings have therefore raised the possibility that other naturally occurring or synthetic coumarins with anti-cancer and anti-inflammatory activities might exert their biological function through the inhibition of MEK1.
Screening of kinase inhibitors targeting BRAF for regulating autophagy based on kinase pathways.[Pubmed:24213221]
Mol Med Rep. 2014 Jan;9(1):83-90.
The aim of this study was to identify agents that regulate autophagy. A total of 544 differentially expressed genes were screened from the intersection set of GSE2435 and GSE31040, which was obtained from the Gene Expression Omnibus database and 19 differentially expressed kinases were selected according to a 'protein kinase database'. Gene ontologybiological process (GO-BP) enrichment analysis revealed that the 19 kinases were mainly associated with phosphorylation. The protein-protein interaction network exhibited 30 differentially expressed genes that interacted with BRAF, and GO-BP enrichment analysis showed the function of these genes were mainly involved in cell death and apoptosis. The kinase-kinase inhibitor regulatory network identified16 kinase inhibitors that specifically inhibited BRAF. Previous studies indicated that sorafenib is capable of regulating autophagy and regorafenib has also been reported; however, there have been no studies regarding the regulation of autophagy by afatinib, selumetinib, PD318088, axitinib, TAK-733, GDC-0980, GSK2126458, PLX-4720, AS703026, trametinib, GDC-0941 and PF-04217903. Thus, these kinase inhibitors are potential targets for further study on the regulation of autophagy in the future.


