Oxyresveratrol 2-O-beta-D-glucopyranosideCAS# 392274-22-5 |
Quality Control & MSDS
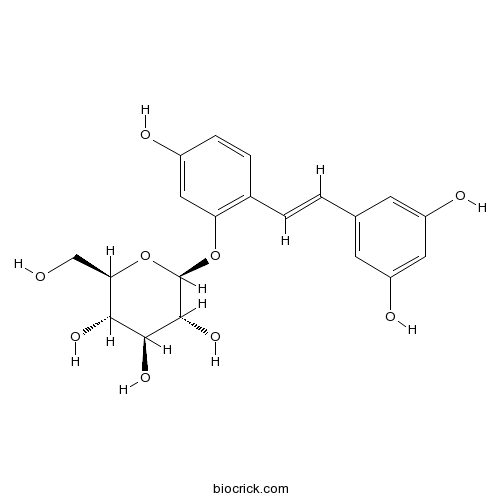
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 392274-22-5 | SDF | Download SDF |
| PubChem ID | 11058597 | Appearance | Powder |
| Formula | C20H22O9 | M.Wt | 406.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[2-[(E)-2-(3,5-dihydroxyphenyl)ethenyl]-5-hydroxyphenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | C1=CC(=C(C=C1O)OC2C(C(C(C(O2)CO)O)O)O)C=CC3=CC(=CC(=C3)O)O | ||
| Standard InChIKey | UPUMEBJDZQEUFC-CUYWLFDKSA-N | ||
| Standard InChI | InChI=1S/C20H22O9/c21-9-16-17(25)18(26)19(27)20(29-16)28-15-8-12(22)4-3-11(15)2-1-10-5-13(23)7-14(24)6-10/h1-8,16-27H,9H2/b2-1+/t16-,17-,18+,19-,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Oxyresveratrol-2-O-beta-D-glucopyranoside shows better tyrosinase inhibitory activities than kojic acid. |
| Targets | NO | Tyrosinase |
| In vitro | Inhibitory effects of constituents from Morus alba var. multicaulis on differentiation of 3T3-L1 cells and nitric oxide production in RAW264.7 cells.[Pubmed: 21772233]Molecules. 2011 Jul 19;16(7):6010-22. doi: 10.3390/molecules16076010.
|
| In vivo | Three major metabolites of mulberroside A in rat intestinal contents and feces.[Pubmed: 19787570]Three major metabolites of mulberroside A in rat intestinal contents and feces.Mulberroside A, a major stilbene constituent of MORUS ALBA L. (Moraceae), displays significant antitussive and antiasthmatic effects in animals. |
| Kinase Assay | Tyrosinase inhibitory constituents from the roots of Morus nigra: a structure-activity relationship study.[Pubmed: 20297841]J Agric Food Chem. 2010 May 12;58(9):5368-73. doi: 10.1021/jf1003607.The phytochemical profiles of Morus nigra roots and twigs were compared by HPLC with those of the old and young twigs of Morus alba which are known to contain oxyresveratrol and mulberroside A as major components. It was found that M. nigra root extract contains some unknown natural products with potential tyrosinase inhibitory activity. |

Oxyresveratrol 2-O-beta-D-glucopyranoside Dilution Calculator

Oxyresveratrol 2-O-beta-D-glucopyranoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4606 mL | 12.3031 mL | 24.6063 mL | 49.2126 mL | 61.5157 mL |
| 5 mM | 0.4921 mL | 2.4606 mL | 4.9213 mL | 9.8425 mL | 12.3031 mL |
| 10 mM | 0.2461 mL | 1.2303 mL | 2.4606 mL | 4.9213 mL | 6.1516 mL |
| 50 mM | 0.0492 mL | 0.2461 mL | 0.4921 mL | 0.9843 mL | 1.2303 mL |
| 100 mM | 0.0246 mL | 0.123 mL | 0.2461 mL | 0.4921 mL | 0.6152 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (E)-Cinnamyl-(Z)-p-coumarate
Catalog No.:BCN7694
CAS No.:391682-51-2
- Ac-His-OH.H2O
Catalog No.:BCC2953
CAS No.:39145-52-3
- Trimebutine
Catalog No.:BCC4615
CAS No.:39133-31-8
- N-Me-Ala-OH.HCl
Catalog No.:BCC2619
CAS No.:3913-67-5
- PD0325901
Catalog No.:BCC1277
CAS No.:391210-10-9
- PD318088
Catalog No.:BCC2539
CAS No.:391210-00-7
- Nigranoic acid
Catalog No.:BCN2399
CAS No.:39111-07-4
- Ajmalan-17-one
Catalog No.:BCN3519
CAS No.:3911-19-1
- Karakoline
Catalog No.:BCC8331
CAS No.:39089-30-0
- UCM 707
Catalog No.:BCC7217
CAS No.:390824-20-1
- Ingenol 20-palmitate
Catalog No.:BCN7678
CAS No.:39071-33-5
- GS 39783
Catalog No.:BCC7233
CAS No.:39069-52-8
- Ginsenoside Compound K
Catalog No.:BCN1246
CAS No.:39262-14-1
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Tiplaxtinin(PAI-039)
Catalog No.:BCC6439
CAS No.:393105-53-8
- 2,3-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN6534
CAS No.:3934-81-4
- BCTC
Catalog No.:BCC7797
CAS No.:393514-24-4
- Neurotensin
Catalog No.:BCC5842
CAS No.:39379-15-2
- Kamebanin
Catalog No.:BCN5449
CAS No.:39388-57-3
- Ethyl 3,4-dihydroxybenzoate
Catalog No.:BCN8504
CAS No.:3943-89-3
- Eleutheroside E
Catalog No.:BCN1083
CAS No.:39432-56-9
- Boceprevir
Catalog No.:BCC1435
CAS No.:394730-60-0
- 3-Acetylcoumarin
Catalog No.:BCC8603
CAS No.:3949-36-8
- CC-401
Catalog No.:BCC4269
CAS No.:395104-30-0
Inhibitory effects of constituents from Morus alba var. multicaulis on differentiation of 3T3-L1 cells and nitric oxide production in RAW264.7 cells.[Pubmed:21772233]
Molecules. 2011 Jul 19;16(7):6010-22.
A new arylbenzofuran, 3',5'-dihydroxy-6-methoxy-7-prenyl-2-arylbenzofuran (1), and 25 known compounds, including moracin R (2), moracin C (3), moracin O (4), moracin P (5), artoindonesianin O (6), moracin D (7), alabafuran A (8), mulberrofuran L (9), mulberrofuran Y (10), kuwanon A (11), kuwanon C (12), kuwanon T (13), morusin (14), kuwanon E (15), sanggenon F (16), betulinic acid (17), uvaol (18), ursolic acid (19), beta-sitosterol (20), Oxyresveratrol 2-O-beta-D-glucopyranoside (21), mulberroside A (22), mulberroside B (23), 5,7-dihydroxycoumarin 7-O-beta-D-glucopyranoside (24), 5,7-dihydroxycoumarin 7-O-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside (25) and adenosine (26), were isolated from Morus alba var. multicaulis Perro. (Moraceae). Their structures were determined by spectroscopic methods. The prenyl-flavonoids 11-14, 16, triterpenoids 17,18 and 20 showed significant inhibitory activity towards the differentiation of 3T3-L1 adipocytes. The arylbenzofurans 1-10 and prenyl-flavonoids 11-16 also showed significant nitric oxide (NO) production inhibitory effects in RAW264.7 cells.
Three major metabolites of mulberroside A in rat intestinal contents and feces.[Pubmed:19787570]
Planta Med. 2010 Mar;76(4):362-4.
Mulberroside A, a major stilbene constituent of MORUS ALBA L. (Moraceae), displays significant antitussive and antiasthmatic effects in animals. As part of our ongoing research on its biotransformation in rats, mulberroside A was orally administered to rats, and the metabolites in the gastrointestinal contents and feces were investigated. Three major metabolites were isolated from the feces of rats and identified as oxyresveratrol-2- O- beta- D-glucopyranoside ( 1), oxyresveratrol-3'- O- beta- D-glucopyranoside ( 2), and oxyresveratrol ( 3) on the basis of chemical and spectroscopic evidence. The three metabolites were also detected in the small intestinal contents of rats following oral administration of mulberroside A. These findings suggest that mulberroside A is metabolized prior to absorption into the body.
Tyrosinase inhibitory constituents from the roots of Morus nigra: a structure-activity relationship study.[Pubmed:20297841]
J Agric Food Chem. 2010 May 12;58(9):5368-73.
The phytochemical profiles of Morus nigra roots and twigs were compared by HPLC with those of the old and young twigs of Morus alba which are known to contain oxyresveratrol and mulberroside A as major components. It was found that M. nigra root extract contains some unknown natural products with potential tyrosinase inhibitory activity. The extract (95% ethanol) of the roots of M. nigra was further investigated in this study. One new compound, 5'-geranyl-5,7,2',4'-tetrahydroxyflavone, and twenty-eight known phenolic compounds were isolated. Their structures were identified by mass spectrometry and NMR spectroscopy. Nine compounds, 5'-geranyl-5,7,2',4'-tetrahydroxyflavone, steppogenin-7-O-beta-D-glucoside, 2,4,2',4'-tetrahydroxychalcone, moracin N, kuwanon H, mulberrofuran G, morachalcone A, oxyresveratrol-3'-O-beta-D-glucopyranoside and oxyresveratrol-2-O-beta-D-glucopyranoside, showed better tyrosinase inhibitory activities than kojic acid. It was noteworthy that the IC(50) values of 2,4,2',4'-tetrahydroxychalcone and morachalcone A were 757-fold and 328-fold lower than that of kojic acid, respectively, suggesting a great potential for their development as effective natural tyrosinase inhibitors.


