Nigranoic acidCAS# 39111-07-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 39111-07-4 | SDF | Download SDF |
| PubChem ID | 10814237 | Appearance | Powder |
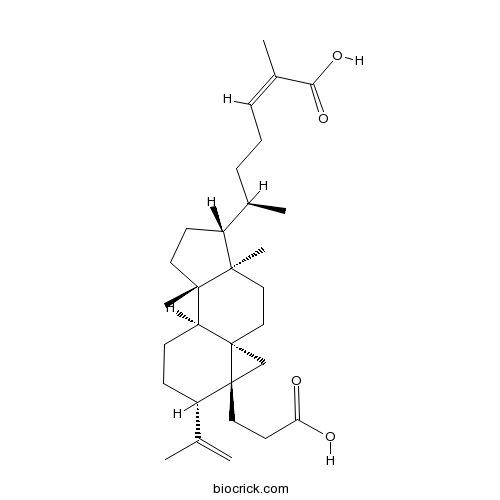
| Formula | C30H46O4 | M.Wt | 470.69 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (Z,6R)-6-[(1S,4R,5R,8S,9S,12S,13R)-13-(2-carboxyethyl)-4,8-dimethyl-12-prop-1-en-2-yl-5-tetracyclo[7.5.0.01,13.04,8]tetradecanyl]-2-methylhept-2-enoic acid | ||
| SMILES | CC(CCC=C(C)C(=O)O)C1CCC2(C1(CCC34C2CCC(C3(C4)CCC(=O)O)C(=C)C)C)C | ||
| Standard InChIKey | NJFOSFIPGRXARF-BRTULJEKSA-N | ||
| Standard InChI | InChI=1S/C30H46O4/c1-19(2)22-10-11-24-28(6)14-12-23(20(3)8-7-9-21(4)26(33)34)27(28,5)16-17-30(24)18-29(22,30)15-13-25(31)32/h9,20,22-24H,1,7-8,10-18H2,2-6H3,(H,31,32)(H,33,34)/b21-9-/t20-,22+,23-,24+,27-,28+,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Nigranoic acid shows activity in several anti-HIV reverse transcriptase and polymerase assays. 2. Nigranoic acid is able to promote NO production and stimulate phosphorylation of ERK1/2 through Ca(2+) influx, further impact expression of BDNF and c-fos, may be benefit to enhance mental and intellectual functions. 3. Nigranoic acid has a strong protective effect on rat cerebral ischemia-reperfusion injury, and acts by downregulating nerve cell apoptosis by preventing the overactivation of PARP and AIF nuclear translocation. 4. Nigranoic acid exhibits significant inhibitory activity against HNE with the IC50 value of 3.77 μM, and six esters displayed considerable inhibitory effects on HNE with IC50 values in the range of 2.61-8.95 μM. |

Nigranoic acid Dilution Calculator

Nigranoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1245 mL | 10.6227 mL | 21.2454 mL | 42.4908 mL | 53.1135 mL |
| 5 mM | 0.4249 mL | 2.1245 mL | 4.2491 mL | 8.4982 mL | 10.6227 mL |
| 10 mM | 0.2125 mL | 1.0623 mL | 2.1245 mL | 4.2491 mL | 5.3114 mL |
| 50 mM | 0.0425 mL | 0.2125 mL | 0.4249 mL | 0.8498 mL | 1.0623 mL |
| 100 mM | 0.0212 mL | 0.1062 mL | 0.2125 mL | 0.4249 mL | 0.5311 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ajmalan-17-one
Catalog No.:BCN3519
CAS No.:3911-19-1
- Karakoline
Catalog No.:BCC8331
CAS No.:39089-30-0
- UCM 707
Catalog No.:BCC7217
CAS No.:390824-20-1
- Ingenol 20-palmitate
Catalog No.:BCN7678
CAS No.:39071-33-5
- GS 39783
Catalog No.:BCC7233
CAS No.:39069-52-8
- Buxbodine D
Catalog No.:BCN5448
CAS No.:390362-53-5
- Buxbodine B
Catalog No.:BCN5447
CAS No.:390362-51-3
- 9-Methoxycamptothecine
Catalog No.:BCN1219
CAS No.:39026-92-1
- Guggulsterone E
Catalog No.:BCC8181
CAS No.:39025-24-6
- Z-Guggulsterone
Catalog No.:BCC7712
CAS No.:39025-23-5
- 3-Epiwilsonine
Catalog No.:BCN5446
CAS No.:39024-15-2
- Wilsonine
Catalog No.:BCN5445
CAS No.:39024-12-9
- PD318088
Catalog No.:BCC2539
CAS No.:391210-00-7
- PD0325901
Catalog No.:BCC1277
CAS No.:391210-10-9
- N-Me-Ala-OH.HCl
Catalog No.:BCC2619
CAS No.:3913-67-5
- Trimebutine
Catalog No.:BCC4615
CAS No.:39133-31-8
- Ac-His-OH.H2O
Catalog No.:BCC2953
CAS No.:39145-52-3
- (E)-Cinnamyl-(Z)-p-coumarate
Catalog No.:BCN7694
CAS No.:391682-51-2
- Oxyresveratrol 2-O-beta-D-glucopyranoside
Catalog No.:BCN1448
CAS No.:392274-22-5
- Ginsenoside Compound K
Catalog No.:BCN1246
CAS No.:39262-14-1
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Tiplaxtinin(PAI-039)
Catalog No.:BCC6439
CAS No.:393105-53-8
- 2,3-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN6534
CAS No.:3934-81-4
- BCTC
Catalog No.:BCC7797
CAS No.:393514-24-4
Synthesis and biological evaluation of nigranoic acid esters as novel human neutrophil elastase inhibitors.[Pubmed:25560928]
Nat Prod Res. 2015;29(17):1650-6.
Human neutrophil elastase (HNE) has been implicated as a major contributor in the pathogenesis of diseases, such as lung disorders and other inflammatory diseases. A series of 12 new Nigranoic acid esters were regioselectively synthesised in good yields and evaluated for HNE inhibitory activity. Nigranoic acid exhibited significant inhibitory activity against HNE with the IC50 value of 3.77 muM, and six esters displayed considerable inhibitory effects on HNE with IC50 values in the range of 2.61-8.95 muM. The Nigranoic acid esters having phenyls substituted with bromine and trimethoxyls (3h and 3b) showed stronger inhibitory activity on HNE than Nigranoic acid.
Nigranoic acid, a triterpenoid from Schisandra sphaerandra that inhibits HIV-1 reverse transcriptase.[Pubmed:8778243]
J Nat Prod. 1996 May;59(5):525-7.
An A ring-secocycloartene triterpenoid, Nigranoic acid (3,4-secocycloarta-4(28),24-(Z)-diene-3,-26-dioic acid, (1) was isolated from the stems of Schisandra sphaerandra, a Chinese traditional medicinal plant. Its structure elucidation and unambiguous NMR spectral assignment were achieved by the combination of 1D- and 2D-NMR techniques with the aid of computer modeling. Nigranoic acid showed activity in several anti-HIV reverse transcriptase and polymerase assays.
Protective effects of nigranoic acid on cerebral ischemia-reperfusion injury and its mechanism involving apoptotic signaling pathway.[Pubmed:25168103]
Cell Biochem Biophys. 2015 Jan;71(1):345-51.
The goal of this study was to assess the expression of poly ADP-ribose polymerase (PARP) and apoptosis-inducing factor (AIF) in the hippocampal CA1 region, and to find out whether Nigranoic acid treatment exhibits protective effects on brain through PARP/AIF signaling pathway in cerebral ischemia-reperfusion animal model. Rats were randomly divided into three groups: Sham-surgery, ischemia-reperfusion, and Nigranoic acid-treated. Rat models of middle cerebral artery occlusion were prepared using a way of thread occlusion. Rats in the Nigranoic acid group were administered with 1 mg/kg intragastric Nigranoic acid 6 and 2 h before brain ischemia, respectively. Following reperfusion, samples were collected at different time-points (6, 24, and 72 h) and each group was further divided into three subgroups. Apoptosis was measured using the terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling method. The protein expression levels of AIF and PARP were detected using Western blot and AIF mRNA quantity was evaluated using the reverse transcription-polymerase chain reaction. Apoptosis, levels of AIF and PARP protein expression, and levels of AIF mRNA expression were significantly increased in the ischemia-reperfusion group compared with the sham-surgery group. However, apoptosis and the expression levels of AIF protein, PARP protein, and AIF mRNA at different time-points were significantly decreased in the Nigranoic acid-treated group compared with the model group. We can judge that Nigranoic acid has a strong protective effect on rat cerebral ischemia-reperfusion injury, and acts by downregulating nerve cell apoptosis by preventing the overactivation of PARP and AIF nuclear translocation.
Effect of nigranoic acid on Ca(2)(+) influx and its downstream signal mechanism in NGF-differentiated PC12 cells.[Pubmed:24674947]
J Ethnopharmacol. 2014 May 14;153(3):725-31.
ETHNOPHARMACOLOGICAL RELEVANCE: Schisandra chinensis has a long history of use as a famous traditional Chinese medicine. The plants of genus Schisandra, especially Schisandra neglecta, Schisandra rubriflora, and Schisandra sphaerandra are used in the same way as Schisandra chinensis in the folk medicine to treat insomnia, fatigue, increasing intelligence, and tranquilizing. Many studies showed that lignans were the major active components of Schisandra genus, whereas the bioactivity of abundant triterpenoids in Schisandra genus, such as Nigranoic acid (SBB1, 3,4-secocycloartene triterpenoid), has not been examined yet in neuropathology. MATERIALS AND METHODS: After treating with SBB1, intracellular Ca(2+) concentration was analyzed by Ca(2+) fluorescent indicator (Fluo-4 AM) in NGF-differentiated PC12 cells. Intracellular nitric oxide (NO) level was analyzed using NO fluorescent indicator (DAF-FM). The expression of extracellular signal regulated kinase 1 and 2 (ERK1/2) was analyzed by western blotting, and the temporal mRNA for BDNF and c-fos was analyzed using reverse transcription quantitative PCR. RESULT: We found that SBB1 induced Ca(2+) influx in a time- and concentration-dependent manner, which was significantly attenuated in Ca(2+) free media. SBB1 promoted the intracellular NO production which depended on increasing cytoplasmic Ca(2+) level. Moreover, SBB1 stimulated activation of ERK1/2 through Ca(2+)-CaMKII pathway. In addition, we found that SBB1 increased the expression of BDNF and c-fos mRNA. CONCLUSION: These results suggest that SBB1 is able to promote NO production and stimulate phosphorylation of ERK1/2 through Ca(2+) influx, further impact expression of BDNF and c-fos, which provides evidence for the effects of SBB1 that may be benefit to enhance mental and intellectual functions.


