Eleutheroside ECAS# 39432-56-9 |
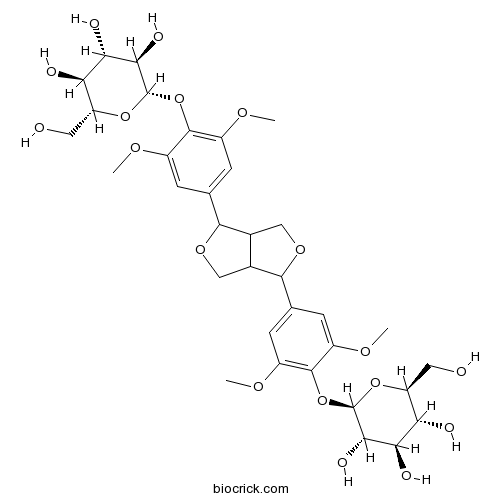
- Liriodendrin
Catalog No.:BCN5774
CAS No.:573-44-4
- Syringaresinol-di-O-glucoside
Catalog No.:BCN2600
CAS No.:66791-77-3
- Eleutheroside D
Catalog No.:BCN5336
CAS No.:79484-75-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 39432-56-9 | SDF | Download SDF |
| PubChem ID | 3084742 | Appearance | White-beige powder |
| Formula | C34H46O18 | M.Wt | 742.73 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 7.5 mg/mL (10.10 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2R,3S,4R,5R,6S)-2-[4-[6-[3,5-dimethoxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-3-yl]-2,6-dimethoxyphenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | COC1=CC(=CC(=C1OC2C(C(C(C(O2)CO)O)O)O)OC)C3C4COC(C4CO3)C5=CC(=C(C(=C5)OC)OC6C(C(C(C(O6)CO)O)O)O)OC | ||
| Standard InChIKey | FFDULTAFAQRACT-RGFZIUCCSA-N | ||
| Standard InChI | InChI=1S/C34H46O18/c1-43-17-5-13(6-18(44-2)31(17)51-33-27(41)25(39)23(37)21(9-35)49-33)29-15-11-48-30(16(15)12-47-29)14-7-19(45-3)32(20(8-14)46-4)52-34-28(42)26(40)24(38)22(10-36)50-34/h5-8,15-16,21-30,33-42H,9-12H2,1-4H3/t15?,16?,21-,22+,23-,24+,25+,26-,27-,28+,29?,30?,33+,34- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Eleutheroside E(EE) has anti-inflammatory and protective effects in ischemia heart, the beneficial effect of EE may provide an effective and powerful strategy to alleviate behavioral alterations induced by sleep deprivation, it may influence to immune-enhancing through increasing the physical endurance capacity and immune cell activation. EE significantly decreases the inflammatory cell infiltration, pannus formation, cartilage damage, bone erosion of CIA mice, the generation of TNF-α and IL-6, the metabolism of drugs metabolized via CYP2C9 and CYP2E1. |
| Targets | NF-kB | TNF-α | IL Receptor | P450 (e.g. CYP17) | IFN-γ |
| In vitro | Immune-enhancing effect of Acanthopanax Koreanum and its component, Eleutheroside E on the protein-energy malnourished C57bl/6 mice[Reference: WebLink]Oriental Pharmacy & Experimental Medicine, 2010, 10(3):191-9.Acanthopanax Koreanum stem (AK) has been used in Korea as a tonic and sedative as well as a drug with ginseng like activities.
|
| In vivo | The effect of Eleutheroside E on behavioral alterations in murine sleep deprivation stress model.[Pubmed: 21376030 ]Eur J Pharmacol. 2011 May 11;658(2-3):150-5.Eleutheroside E (EE), a principal component of Eleutherococcus senticosus, has been reported to have anti-inflammatory and protective effects in ischemia heart etc. However, whether it can mitigate behavioral alterations induced by sleep deprivation, has not yet been elucidated. Numerous studies have demonstrated that memory deficits induced by sleep deprivation in experimental animals can be used as a model of behavioral alterations.
The present study investigated the effect of EE, on cognitive performances and biochemical parameters of sleep-deprived mice.
|
| Cell Research | Effects of eleutheroside B and eleutheroside E on activity of cytochrome P450 in rat liver microsomes.[Pubmed: 24383621]BMC Complement Altern Med. 2014 Jan 2;14:1.Chemicals of herbal products may cause unexpected toxicity or adverse effect by the potential for alteration of the activity of CYP450 when co-administered with other drugs. Eleutherococcus senticosus (ES), has been widely used as a traditional herbal medicine and popular herbal dietary supplements, and often co-administered with many other drugs. The main bioactive constituents of ES were considered to be eleutherosides including eleutheroside B (EB) and Eleutheroside E (EE). This study was to investigate the effects of EB and EE on CYP2C9, CYP2D6, CYP2E1 and CYP3A4 in rat liver microsomes in vitro.
|
| Animal Research | Eleutheroside E ameliorates arthritis severity in collagen-induced arthritis mice model by suppressing inflammatory cytokine release.[Pubmed: 24917466]Inflammation. 2014 Oct;37(5):1533-43.Rheumatoid arthritis is the most common arthritis and is mainly characterized by symmetric polyarticular joint disorders. Eleutheroside E (EE), a principal active constituent of Acanthopanax senticosus, is reported to have anti-inflammatory effect by inhibiting NF-κB activities. However, the effects of EE on rheumatoid arthritis (RA) severity are largely unknown.
|
| Structure Identification | Arch Pharm Res. 2015 Nov;38(11):1921-5.Utilization of circular dichroism experiment to distinguish acanthoside D and eleutheroside E.[Pubmed: 25802110 ]Two lignan glycosides, acanthoside D (1) (=liriodendrin, (+)-syringaresinol di-O-β-D-glucopyranoside) and Eleutheroside E (2) have been confused each other for so long time, and hard to be distinguished each other.
Now, this two compounds need to be defined properly so that all the commercial mistakes and confusions should not be made.
|

Eleutheroside E Dilution Calculator

Eleutheroside E Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3464 mL | 6.7319 mL | 13.4638 mL | 26.9277 mL | 33.6596 mL |
| 5 mM | 0.2693 mL | 1.3464 mL | 2.6928 mL | 5.3855 mL | 6.7319 mL |
| 10 mM | 0.1346 mL | 0.6732 mL | 1.3464 mL | 2.6928 mL | 3.366 mL |
| 50 mM | 0.0269 mL | 0.1346 mL | 0.2693 mL | 0.5386 mL | 0.6732 mL |
| 100 mM | 0.0135 mL | 0.0673 mL | 0.1346 mL | 0.2693 mL | 0.3366 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Eleutheroside E, a principal component of Eleutherococcus enticosus, has anti-inflammatory and protective effects in ischemia heart. IC50 value: Target: In vitro: Treatment of 10 μM Eleutheroside E (EE) for 24 h increased basal glucose uptake as well as improved TNF-α-mediated suppression of glucose uptake. [2] In vivo: To investigate the effect of Eleutheroside E (EE) on arthritis, the CIA model in DBA/1 mice was used. Compared to vehicle-treated CIA mice, 15 mg/kg TG treatment and 30 and 60 mg/kg EE treatment obviously decreased the arthritis scores and body weight loss in CIA mice (P<0.01) [1].
References:
[1]. Chunyan He, et al. Eleutheroside E Ameliorates Arthritis Severity in Collagen-Induced Arthritis Mice Model by Suppressing Inflammatory Cytokine Release. Inflammation, Vol. 37, No. 5, October 2014 (# 2014)
[2]. Jiyun Ahn, et al. Eleutheroside E, An Active Component of Eleutherococcus senticosus, Ameliorates Insulin Resistance in Type 2 Diabetic db/db Mice. Evid Based Complement Alternat Med. 2013; 2013: 934183.
- Ethyl 3,4-dihydroxybenzoate
Catalog No.:BCN8504
CAS No.:3943-89-3
- Kamebanin
Catalog No.:BCN5449
CAS No.:39388-57-3
- Neurotensin
Catalog No.:BCC5842
CAS No.:39379-15-2
- BCTC
Catalog No.:BCC7797
CAS No.:393514-24-4
- 2,3-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN6534
CAS No.:3934-81-4
- Tiplaxtinin(PAI-039)
Catalog No.:BCC6439
CAS No.:393105-53-8
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Ginsenoside Compound K
Catalog No.:BCN1246
CAS No.:39262-14-1
- Oxyresveratrol 2-O-beta-D-glucopyranoside
Catalog No.:BCN1448
CAS No.:392274-22-5
- (E)-Cinnamyl-(Z)-p-coumarate
Catalog No.:BCN7694
CAS No.:391682-51-2
- Ac-His-OH.H2O
Catalog No.:BCC2953
CAS No.:39145-52-3
- Trimebutine
Catalog No.:BCC4615
CAS No.:39133-31-8
- Boceprevir
Catalog No.:BCC1435
CAS No.:394730-60-0
- 3-Acetylcoumarin
Catalog No.:BCC8603
CAS No.:3949-36-8
- CC-401
Catalog No.:BCC4269
CAS No.:395104-30-0
- Sinigrin
Catalog No.:BCN8484
CAS No.:3952-98-5
- Asperosaponin VI
Catalog No.:BCN1256
CAS No.:39524-08-8
- Nitrendipine
Catalog No.:BCC4381
CAS No.:39562-70-4
- Triamterene
Catalog No.:BCC5074
CAS No.:396-01-0
- Z-Sar-OH
Catalog No.:BCC3339
CAS No.:39608-31-6
- Pasireotide
Catalog No.:BCC5300
CAS No.:396091-73-9
- LY364947
Catalog No.:BCC5085
CAS No.:396129-53-6
- Isoshinanolone
Catalog No.:BCN7986
CAS No.:39626-91-0
- Azatadine
Catalog No.:BCC4133
CAS No.:3964-81-6
Effects of eleutheroside B and eleutheroside E on activity of cytochrome P450 in rat liver microsomes.[Pubmed:24383621]
BMC Complement Altern Med. 2014 Jan 2;14:1.
BACKGROUND: Chemicals of herbal products may cause unexpected toxicity or adverse effect by the potential for alteration of the activity of CYP450 when co-administered with other drugs. Eleutherococcus senticosus (ES), has been widely used as a traditional herbal medicine and popular herbal dietary supplements, and often co-administered with many other drugs. The main bioactive constituents of ES were considered to be eleutherosides including eleutheroside B (EB) and Eleutheroside E (EE). This study was to investigate the effects of EB and EE on CYP2C9, CYP2D6, CYP2E1 and CYP3A4 in rat liver microsomes in vitro. METHOD: Probe drugs of tolbutamide (TB), dextromethorphan (DM), chlorzoxazone (CLZ) and testosterone (TS) as well as eleutherosides of different concentrations were added to incubation systems of rat liver microsomes in vitro. After incubation, validated HPLC methods were used to quantify relevant metabolites. RESULTS: The results suggested that EB and EE exhibited weak inhibition against the activity of CYP2C9 and CYP2E1, but no effects on CYP2D6 and CYP3A4 activity. The IC50 values for EB and EE were calculated to be 193.20 muM and 188.36 muM for CYP2E1, 595.66 muM and 261.82 muM for CYP2C9, respectively. Kinetic analysis showed that inhibitions of CYP2E1 by EB and EE were best fit to mixed-type with Ki value of 183.95 muM and 171.63 muM, respectively. CONCLUSIONS: These results indicate that EB and EE may inhibit the metabolism of drugs metabolized via CYP2C9 and CYP2E1, and have the potential to increase the toxicity of the drugs.
Utilization of circular dichroism experiment to distinguish acanthoside D and eleutheroside E.[Pubmed:25802110]
Arch Pharm Res. 2015 Nov;38(11):1921-5.
Two lignan glycosides, acanthoside D (1) (=liriodendrin, (+)-syringaresinol di-O-beta-D-glucopyranoside) and Eleutheroside E (2) have been confused each other for so long time, and hard to be distinguished each other. Now, this two compounds need to be defined properly so that all the commercial mistakes and confusions should not be made. They have identical planar structures except for the configurations at C-7 and C-8 in each structure according to the chemistry database, SciFinder((R)). The systematic name of acanthoside D is [(1S,3aR,4S,6aR)-tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diyl]bis(2,6-dimethoxy-4,1 -phenylene) bis-beta-D-glucopyranoside (1), and the name of Eleutheroside E is [(1R,3aR,4S,6aS)-tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diyl]bis(2,6-dimethoxy-4,1 -phenylene) bis-beta-D-glucopyranoside (2). The differences at two chiral centers do not make any differences in the NMR spectra. Thus, the circular dichroism were utilized to dissolve this difficult problem. Acanthoside D (1) showed a positive Cotton effect at 200 nm, whereas Eleutheroside E (2) exhibited a negative cotton effect at 200 nm. The absolute structure of acanthoside D was also confirmed by X-ray crystallography.
The effect of Eleutheroside E on behavioral alterations in murine sleep deprivation stress model.[Pubmed:21376030]
Eur J Pharmacol. 2011 May 11;658(2-3):150-5.
Eleutheroside E (EE), a principal component of Eleutherococcus senticosus, has been reported to have anti-inflammatory and protective effects in ischemia heart etc. However, whether it can mitigate behavioral alterations induced by sleep deprivation, has not yet been elucidated. Numerous studies have demonstrated that memory deficits induced by sleep deprivation in experimental animals can be used as a model of behavioral alterations. The present study investigated the effect of EE, on cognitive performances and biochemical parameters of sleep-deprived mice. Animals were repeatedly treated with saline, 10 or 50mg/kg EE and sleep-deprived for 72 h by the multiple platform method. Briefly, groups of 5-6 mice were placed in water tanks (45 x 34 x 17 cm), containing 12 platforms (3 cm in diameter) each, surrounded by water up to 1cm beneath the surface or kept in their home cage. After sleep deprivation, mice showed significant behavioral impairment as evident by reduced latency entering into a dark chamber, locomotion and correctly rate in Y maze, and increased monoamines in hippocampus. However, repeated treatment with EE restored these behavioral and biochemical alterations in mice. In conclusion, the beneficial effect of EE may provide an effective and powerful strategy to alleviate behavioral alterations induced by sleep deprivation.
Eleutheroside E ameliorates arthritis severity in collagen-induced arthritis mice model by suppressing inflammatory cytokine release.[Pubmed:24917466]
Inflammation. 2014 Oct;37(5):1533-43.
Rheumatoid arthritis is the most common arthritis and is mainly characterized by symmetric polyarticular joint disorders. Eleutheroside E (EE), a principal active constituent of Acanthopanax senticosus, is reported to have anti-inflammatory effect by inhibiting NF-kappaB activities. However, the effects of EE on rheumatoid arthritis (RA) severity are largely unknown. The purpose of this study was to indicate whether EE could ameliorate arthritis and reduce inflammatory cytokine release in collagen-induced arthritis (CIA) mice. The results showed that EE attenuated the severity of arthritis by reducing the mean arthritis score and arthritis incidence. EE also significantly decreased the inflammatory cell infiltration, pannus formation, cartilage damage, and bone erosion of CIA mice. Furthermore, EE caused a marked decrease of the production of TNF-alpha and IL-6 in vivo and in vitro. These observations identify a novel function of EE that results in inhibition of cytokine release, highlighting EE was a potential therapeutic agent for RA.


