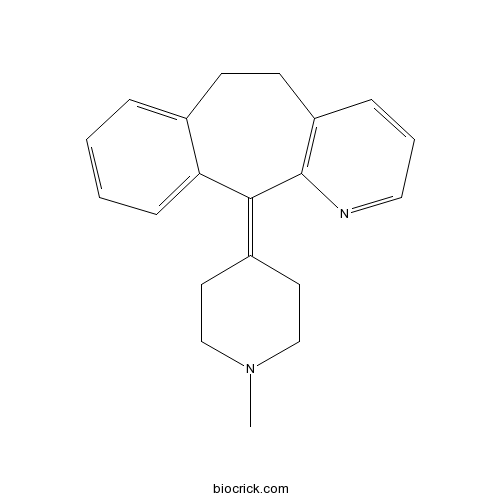
AzatadineCAS# 3964-81-6 |
- Milnacipran HCl
Catalog No.:BCC4922
CAS No.:101152-94-7
- Escitalopram
Catalog No.:BCC4193
CAS No.:128196-01-0
- Escitalopram Oxalate
Catalog No.:BCC5040
CAS No.:219861-08-2
- Sertraline HCl
Catalog No.:BCC5059
CAS No.:79559-97-0
- Milnacipran
Catalog No.:BCC4194
CAS No.:92623-85-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3964-81-6 | SDF | Download SDF |
| PubChem ID | 19861 | Appearance | Powder |
| Formula | C20H22N2 | M.Wt | 290.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | 11-(1-methylpiperidin-4-ylidene)-5,6-dihydrobenzo[1,2]cyclohepta[3,4-b]pyridine | ||
| SMILES | CN1CCC(=C2C3=CC=CC=C3CCC4=C2N=CC=C4)CC1 | ||
| Standard InChIKey | SEBMTIQKRHYNIT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H22N2/c1-22-13-10-16(11-14-22)19-18-7-3-2-5-15(18)8-9-17-6-4-12-21-20(17)19/h2-7,12H,8-11,13-14H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Azatadine Dilution Calculator

Azatadine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4435 mL | 17.2176 mL | 34.4353 mL | 68.8705 mL | 86.0882 mL |
| 5 mM | 0.6887 mL | 3.4435 mL | 6.8871 mL | 13.7741 mL | 17.2176 mL |
| 10 mM | 0.3444 mL | 1.7218 mL | 3.4435 mL | 6.8871 mL | 8.6088 mL |
| 50 mM | 0.0689 mL | 0.3444 mL | 0.6887 mL | 1.3774 mL | 1.7218 mL |
| 100 mM | 0.0344 mL | 0.1722 mL | 0.3444 mL | 0.6887 mL | 0.8609 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Azatadine is an histamine and cholinergic inhibitor with IC50 of 6.5 nM and 10 nM, respectively.
- Isoshinanolone
Catalog No.:BCN7986
CAS No.:39626-91-0
- LY364947
Catalog No.:BCC5085
CAS No.:396129-53-6
- Pasireotide
Catalog No.:BCC5300
CAS No.:396091-73-9
- Z-Sar-OH
Catalog No.:BCC3339
CAS No.:39608-31-6
- Triamterene
Catalog No.:BCC5074
CAS No.:396-01-0
- Nitrendipine
Catalog No.:BCC4381
CAS No.:39562-70-4
- Asperosaponin VI
Catalog No.:BCN1256
CAS No.:39524-08-8
- Sinigrin
Catalog No.:BCN8484
CAS No.:3952-98-5
- CC-401
Catalog No.:BCC4269
CAS No.:395104-30-0
- 3-Acetylcoumarin
Catalog No.:BCC8603
CAS No.:3949-36-8
- Boceprevir
Catalog No.:BCC1435
CAS No.:394730-60-0
- Eleutheroside E
Catalog No.:BCN1083
CAS No.:39432-56-9
- BVT 948
Catalog No.:BCC2467
CAS No.:39674-97-0
- (R)-Reticuline
Catalog No.:BCN6795
CAS No.:3968-19-2
- 1,5-Diphenylpentan-1-one
Catalog No.:BCN7169
CAS No.:39686-51-6
- 2-Benzylsuccinic acid
Catalog No.:BCC8566
CAS No.:3972-36-9
- Catharticin
Catalog No.:BCN6850
CAS No.:39723-40-5
- Daphmacropodine
Catalog No.:BCN5450
CAS No.:39729-21-0
- Gue 1654
Catalog No.:BCC6274
CAS No.:397290-30-1
- SDZ 21009
Catalog No.:BCC7098
CAS No.:39731-05-0
- H-Gln-OtBu.HCl
Catalog No.:BCC2918
CAS No.:39741-62-3
- 16,16-Dimethyl Prostaglandin E2
Catalog No.:BCC7843
CAS No.:39746-25-3
- Dehydrovomifoliol
Catalog No.:BCN7562
CAS No.:39763-33-2
- Bryonolol
Catalog No.:BCN2703
CAS No.:39765-50-9
Determination of azatadine in human plasma by liquid chromatography/tandem mass spectrometry.[Pubmed:21737359]
J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Aug 1;879(23):2189-93.
A sensitive method using liquid chromatography with tandem mass spectrometric detection (LC-MS/MS) was developed and validated for the analysis of antihistamine drug Azatadine in human plasma. Loratadine was used as internal standard (IS). Analytes were extracted from human plasma by liquid/liquid extraction using ethyl acetate. The organic phase was reduced to dryness under a stream of nitrogen at 30 degrees C and the residue was reconstituted with the mobile phase. 5 muL of the resulting solution was injected onto the LC-MS/MS system. A 4.6 mm x 150 mm, I.D. 5 mum, Agilent TC-C(18) column was used to perform the chromatographic analysis. The mobile phase consisted of ammonium formate buffer 0.010 M (adjusted to pH 4.3 with 1M formic acid)/acetonitrile (20:80, v/v) The chromatographic run time was 5 min per injection and flow rate was 0.6 mL/min. The retention time was 2.4 and 4.4 min for Azatadine and IS, respectively. The tandem mass spectrometric detection mode was achieved with electrospray ionization (ESI) iron source and the multiple reaction monitoring (MRM) (291.3 --> 248.2m/z for Azatadine, 383.3 --> 337.3m/z for IS) was operated in positive ion modes. The low limit of quantitation (LLOQ) was 0.05 ng/mL. The intra-day and inter-day precision of the quality control (QC) samples was 8.93-11.57% relative standard deviation (RSD). The inter-day accuracy of the QC samples was 96.83-105.07% of the nominal values.
Syntheses of molecularly imprinted polymers: Molecular recognition of cyproheptadine using original print molecules and azatadine as dummy templates.[Pubmed:19084632]
Anal Chim Acta. 2009 Jan 12;631(2):237-44.
The use of custom-made polymeric materials with high selectivities as target molecules in solid-phase extraction (SPE), known as molecularly imprinted solid-phase extraction (MISPE), is becoming an increasingly important sample preparation technique. However, the potential risk of leakage of the imprinting molecules during the desorption phase has limited application. The use of a mimicking template, called a dummy molecular imprinting polymer (DMIP), that bears the structure of a related molecule and acts as a putative imprinting molecule may provide a useful solution to this problem. In the current study, cyproheptadine (CPH) and Azatadine (AZA) were used as templates in the development of an MIP and DMIP for acrylic acid and methacrylic acid monomers. Our results indicate that DMIPs have equal recognition of CPH, avoiding the problem of leakage of original template during the desorption phase relative to MIPs synthesized in presence of the print molecule CPH. Examination of the surface structure of the two polymer products by SEM shows appreciable differences in structural morphology and function of the monomers employed. These results are well supplemented by data obtained for swelling ratios and solvent uptake. Molecular modelling of CPH and AZA suggests that both substrates are similar in shape and volume.
Fungal biotransformation of the antihistamine azatadine by Cunninghamella elegans.[Pubmed:8795241]
Appl Environ Microbiol. 1996 Sep;62(9):3477-9.
The metabolism of the antihistamine Azatadine by the zygomycete fungus Cunninghamella elegans ATCC 9245 was investigated. Within 72 h from the addition of the drug to 48-h-old cultures grown in Sabouraud dextrose broth, 95% of Azatadine was biotransformed. Two major metabolites, 7-hydroxyAzatadine (25%) and 8-hydroxyAzatadine (50%), and two minor metabolites, N-desmethylAzatadine and 9-hydroxyAzatadine, were isolated by high-performance liquid chromatography and characterized by mass spectrometric and proton nuclear magnetic resonance spectroscopic analyses.
Comparative effects of loratadine and azatadine in the treatment of seasonal allergic rhinitis.[Pubmed:1982614]
Asian Pac J Allergy Immunol. 1990 Dec;8(2):103-7.
The efficacy and safety of loratadine 10 mg once daily were compared with Azatadine 1 mg twice daily in controlling symptoms of seasonal allergic rhinitis. The study was a randomized, double-blind, parallel-group design involving 34 patients receiving either loratadine or Azatadine for 14 days. Both treatments were effective in relieving the histamine-mediated symptoms of seasonal allergic rhinitis. At baseline, 100% and 93% of the patients in the loratadine and Azatadine treatment groups, respectively, had moderate or severe symptoms of disease; at endpoint of treatment 80% of the patients in the loratadine treatment group and 92% of those in the Azatadine treatment group had mild or no disease symptoms. Sedation was reported by fewer patients in the loratadine treatment group than in the Azatadine group. Thus loratadine is an effective and safe antihistamine when given once daily for the symptomatic relief of seasonal allergic rhinitis.


