LY364947inhibitor of TGF-β type I receptor kinase domain CAS# 396129-53-6 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- CTEP (RO4956371)
Catalog No.:BCC4599
CAS No.:871362-31-1
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
- MPEP
Catalog No.:BCC4594
CAS No.:96206-92-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 396129-53-6 | SDF | Download SDF |
| PubChem ID | 447966 | Appearance | Powder |
| Formula | C17H12N4 | M.Wt | 272.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | HTS 466284 | ||
| Solubility | DMSO : 25 mg/mL (91.81 mM; Need ultrasonic and warming) H2O : < 0.1 mg/mL (insoluble) | ||
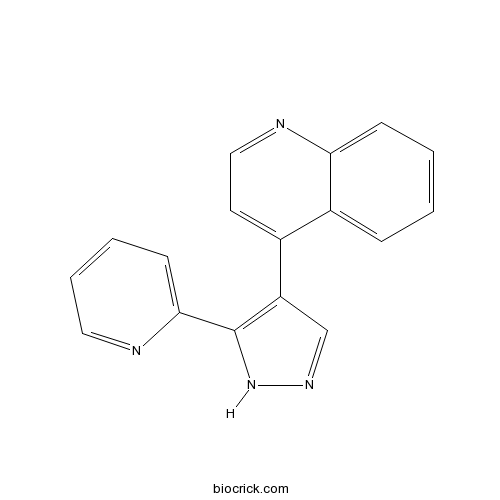
| Chemical Name | 4-(5-pyridin-2-yl-1H-pyrazol-4-yl)quinoline | ||
| SMILES | C1=CC=C2C(=C1)C(=CC=N2)C3=C(NN=C3)C4=CC=CC=N4 | ||
| Standard InChIKey | IBCXZJCWDGCXQT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H12N4/c1-2-6-15-13(5-1)12(8-10-19-15)14-11-20-21-17(14)16-7-3-4-9-18-16/h1-11H,(H,20,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of TGF-β type-I receptor (TGF-β RI, TGFR-I, TβR-I, ALK-5) (IC50 values are 59, 400 and 1400 nM for TGR-β RI, TGF-β RII and MLK-7K respectively). Inhibits TGF-β-dependent luciferase production in mink lung cells (p3TP lux) and growth in mouse fibroblasts (NIH 3T3) (IC50 values are 47 and 89 nM respectively). Suppresses invasion of MDA-MB-231 breast cancer cells in a matrigel invasion assay. |

LY364947 Dilution Calculator

LY364947 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6724 mL | 18.3621 mL | 36.7242 mL | 73.4484 mL | 91.8105 mL |
| 5 mM | 0.7345 mL | 3.6724 mL | 7.3448 mL | 14.6897 mL | 18.3621 mL |
| 10 mM | 0.3672 mL | 1.8362 mL | 3.6724 mL | 7.3448 mL | 9.1811 mL |
| 50 mM | 0.0734 mL | 0.3672 mL | 0.7345 mL | 1.469 mL | 1.8362 mL |
| 100 mM | 0.0367 mL | 0.1836 mL | 0.3672 mL | 0.7345 mL | 0.9181 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
The multifunctional cytokine transforming growth factor-β (TGF-β) is a member of a large family of growth factors involved in the regulation of a diverse array of biological processes including cell growth and differentiation, matrix modulation, and embryonic development. LY364947 is a inhibitor of the transforming growth factor-β type I receptor kinase domain.
In vitro: LY364947 was quickly identified as a potent inhibitor (IC50= 51 nM) and was chosen as a platform for SAR development. Compounds were further evaluated as inhibitors of TGF-β-dependent luciferase production in mink lung cells (p3TP Lux) and growth in mouse fibroblasts (NIH 3T3) [1].
In vivo: In a rat model of NMDA-induced retinal degeneration, simultaneous injection of NMDA and the TGF-β inhibitor LY364947 slightly but significantly attenuated the reduction in number of cells in the ganglion cell layer and almost completely prevented the enhancement of capillary degeneration. [3].
Clinical trial: Up to now, LY364947 is still in the preclinical development stage.
Reference:
[1] Sawyer JS, Anderson BD, Beight DW, Campbell RM, Jones ML, Herron DK, Lampe JW, McCowan JR, McMillen WT, Mort N, Parsons S, Smith EC, Vieth M, Weir LC, Yan L, Zhang F, Yingling JM. Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J Med Chem. 2003 Sep 11;46(19):3953-6.
[2] Ueda K, Nakahara T, Mori A, Sakamoto K, Ishii K. Protective effects of TGF-β inhibitors in a rat model of NMDA-induced retinal degeneration. Eur J Pharmacol. 2013 Jan 15;699(1-3):188-93.
- Pasireotide
Catalog No.:BCC5300
CAS No.:396091-73-9
- Z-Sar-OH
Catalog No.:BCC3339
CAS No.:39608-31-6
- Triamterene
Catalog No.:BCC5074
CAS No.:396-01-0
- Nitrendipine
Catalog No.:BCC4381
CAS No.:39562-70-4
- Asperosaponin VI
Catalog No.:BCN1256
CAS No.:39524-08-8
- Sinigrin
Catalog No.:BCN8484
CAS No.:3952-98-5
- CC-401
Catalog No.:BCC4269
CAS No.:395104-30-0
- 3-Acetylcoumarin
Catalog No.:BCC8603
CAS No.:3949-36-8
- Boceprevir
Catalog No.:BCC1435
CAS No.:394730-60-0
- Eleutheroside E
Catalog No.:BCN1083
CAS No.:39432-56-9
- Ethyl 3,4-dihydroxybenzoate
Catalog No.:BCN8504
CAS No.:3943-89-3
- Kamebanin
Catalog No.:BCN5449
CAS No.:39388-57-3
- Isoshinanolone
Catalog No.:BCN7986
CAS No.:39626-91-0
- Azatadine
Catalog No.:BCC4133
CAS No.:3964-81-6
- BVT 948
Catalog No.:BCC2467
CAS No.:39674-97-0
- (R)-Reticuline
Catalog No.:BCN6795
CAS No.:3968-19-2
- 1,5-Diphenylpentan-1-one
Catalog No.:BCN7169
CAS No.:39686-51-6
- 2-Benzylsuccinic acid
Catalog No.:BCC8566
CAS No.:3972-36-9
- Catharticin
Catalog No.:BCN6850
CAS No.:39723-40-5
- Daphmacropodine
Catalog No.:BCN5450
CAS No.:39729-21-0
- Gue 1654
Catalog No.:BCC6274
CAS No.:397290-30-1
- SDZ 21009
Catalog No.:BCC7098
CAS No.:39731-05-0
- H-Gln-OtBu.HCl
Catalog No.:BCC2918
CAS No.:39741-62-3
- 16,16-Dimethyl Prostaglandin E2
Catalog No.:BCC7843
CAS No.:39746-25-3
Arsenic-induced interstitial myocardial fibrosis reveals a new insight into drug-induced long QT syndrome.[Pubmed:22853924]
Cardiovasc Res. 2012 Oct 1;96(1):90-8.
AIMS: Arsenic trioxide (ATO), an effective therapeutic agent for acute promyelocytic leukaemia, can cause sudden cardiac death due to long QT syndrome (LQTS). The present study was designed to determine whether ATO could induce cardiac fibrosis and explore whether cardiac fibroblasts (CFs) are involved in the development of LQTS by ATO. METHODS AND RESULTS: ATO treatment of guinea pigs caused substantial interstitial myocardial fibrosis and LQTS, which was accompanied by an increase in transforming growth factor beta1(TGF-beta1) secretion and a decrease in ether-a-go-go-related gene (HERG) and inward rectifying potassium channel (I(K1)) subunit Kir2.1 protein levels. ATO promoted collagen production and TGF-beta1 expression and secretion in cultured CFs. Whole-cell patch clamp and western blotting showed that treatment with TGF-beta1 markedly reduced HERG and I(K1) current densities and downregulated HERG and Kir2.1 protein expression in HEK293 cells stably transfected with the human recombinant HERG channel and in cardiomyocytes (CMs). These changes were completely reversed by treatment with the protein kinase A (PKA) antagonist, H89. CM and CF co-cultures showed that ATO significantly increased TGF-beta1 levels in the culture medium, whereas markedly reduced HERG and Kir2.1 protein levels were observed in CMs compared with ATO-treated CMs not co-cultured with CFs. Finally, in vivo administration of LY364947, a pharmacological antagonist of TGF-beta signalling, dramatically prevented interstitial fibrosis and LQTS and abolished aberrant expression of TGF-beta1, HERG, and Kir2.1 in ATO-treated guinea pigs. CONCLUSION: ATO-induced TGF-beta1 secretion from CFs aggravates QT prolongation, suggesting that modulation of TGF-beta signalling may provide a novel strategy for the treatment of drug-induced LQTS.
Attenuation of the DNA damage response by transforming growth factor-beta inhibitors enhances radiation sensitivity of non-small-cell lung cancer cells in vitro and in vivo.[Pubmed:25835621]
Int J Radiat Oncol Biol Phys. 2015 Jan 1;91(1):91-9.
PURPOSE: To determine whether transforming growth factor (TGF)-beta inhibition increases the response to radiation therapy in human and mouse non-small-cell lung carcinoma (NSCLC) cells in vitro and in vivo. METHODS AND MATERIALS: TGF-beta-mediated growth response and pathway activation were examined in human NSCLC NCI-H1299, NCI-H292, and A549 cell lines and murine Lewis lung cancer (LLC) cells. Cells were treated in vitro with LY364947, a small-molecule inhibitor of the TGF-beta type 1 receptor kinase, or with the pan-isoform TGF-beta neutralizing monoclonal antibody 1D11 before radiation exposure. The DNA damage response was assessed by ataxia telangiectasia mutated (ATM) or Trp53 protein phosphorylation, gammaH2AX foci formation, or comet assay in irradiated cells. Radiation sensitivity was determined by clonogenic assay. Mice bearing syngeneic subcutaneous LLC tumors were treated with 5 fractions of 6 Gy and/or neutralizing or control antibody. RESULTS: The NCI-H1299, A549, and LLC NSCLC cell lines pretreated with LY364947 before radiation exposure exhibited compromised DNA damage response, indicated by decreased ATM and p53 phosphorylation, reduced gammaH2AX foci, and increased radiosensitivity. The NCI-H292 cells were unresponsive. Transforming growth factor-beta signaling inhibition in irradiated LLC cells resulted in unresolved DNA damage. Subcutaneous LLC tumors in mice treated with TGF-beta neutralizing antibody exhibited fewer gammaH2AX foci after irradiation and significantly greater tumor growth delay in combination with fractionated radiation. CONCLUSIONS: Inhibition of TGF-beta before radiation attenuated DNA damage recognition and increased radiosensitivity in most NSCLC cells in vitro and promoted radiation-induced tumor control in vivo. These data support the rationale for concurrent TGF-beta inhibition and RT to provide therapeutic benefit in NSCLC.
Characterization of drug-lysozyme conjugates by sheathless capillary electrophoresis-time-of-flight mass spectrometry.[Pubmed:21645662]
Anal Chim Acta. 2011 Jul 18;698(1-2):77-83.
Drug-protein conjugates have been widely used for the cell-specific targeting of drugs to cells that can bind and internalize the proteinaceous carrier. For renal drug targeting, lysozyme (LZM) can be used as an effective carrier that accumulates in proximal tubular cells. We used capillary electrophoresis-time-of-flight mass spectrometry (CE-TOF-MS) for the characterization of different drug-LZM conjugates. A recently developed prototype porous tip sprayer was employed for sheathless electrospray ionization (ESI) CE-MS interfacing. In order to prevent adsorption of LZM conjugates to the capillary wall, a positively charged polyethylenimine capillary coating was used in combination with a low-pH background electrolyte. Drug-LZM products had been prepared by first coupling BOC-l-methionine hydroxysuccinimide ester (BOCmet) to lysine residues of LZM followed by conjugation with the kinase inhibitors LY364947, erlotinib, or Y27632 via a platinum(II)-based linker. CE-TOF-MS of each preparation showed narrow symmetrical peaks for the various reaction products demonstrating that drug-LZM conjugates remained stable during the CE analysis and subsequent ESI. Components observed in the drug-LZM products were assigned based on their relative migration times and on molecular mass as obtained by TOF-MS. The TOF-MS data obtained for the individual components revealed that the preparations contained LZM carrying one or two drug molecules, next to unmodified and BOCmet-modified LZM. Based on relative peak areas (assuming an equimolar response for each component) a quantitative conjugate profile could be derived for every preparation leading to drug loading values of 0.4-0.6 mol drug per mole protein.
Arsenic trioxide induces cardiac fibroblast apoptosis in vitro and in vivo by up-regulating TGF-beta1 expression.[Pubmed:23542815]
Toxicol Lett. 2013 Jun 7;219(3):223-30.
Arsenic trioxide (As2O3; ATO) is clinically effective in treating acute promyelocytic leukemia (APL); however, it frequently causes cardiotoxic effects. This study was designed to investigate whether ATO could induce apoptosis of cardiac fibroblasts (CFs) that play very important roles in maintaining the structure integrity and function of the heart. Cardiac fibroblasts from guinea pigs administered with ATO (1mg/kgbw) were used to test the pro-apoptotic role of ATO in vivo. The current study demonstrated that ATO induced morphological characteristics of apoptosis and Caspase-3 activation in CFs of guinea pigs along with a significant up-regulation in TGF-beta1 protein expression, Bax/Bcl-2 ratio and ERK1/2 phosphorylation. In vitro MTT assay showed that ATO remarkably reduced the viability of cultured cardiac fibroblasts (NRCFs) from neonatal rat in a concentration- and time-dependent manner. Consistent with the notions in vivo, ATO significantly induced the apoptosis in NRCFs, dramatically up-regulated TGF-beta1 protein level and Bax/Bcl-2 ratio in a time-dependent fashion and activated Caspase-3 and ERK1/2. Finally, pretreatment with LY364947, an inhibitor of TGF-beta signaling could apparently reverse these changes. We therefore conclude that TGF-beta is functionally linked to ERK1/2 and that TGF-beta signaling is responsible for ATO-induced CFs apoptosis, which provides a novel mechanism of ATO related cardiac toxicology.
Combined treatment with erlotinib and a transforming growth factor-beta type I receptor inhibitor effectively suppresses the enhanced motility of erlotinib-resistant non-small-cell lung cancer cells.[Pubmed:23334091]
J Thorac Oncol. 2013 Mar;8(3):259-69.
INTRODUCTION: : Despite an initial dramatic response to the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib, the majority of non-small cell lung cancer (NSCLC) patients with EGFR-activating mutations develop acquired resistance. Therefore, there is an urgent need to elucidate the unknown mechanisms and biological behaviors of EGFR TKI-resistant lung tumors. We investigated the motility of EGFR TKI-resistant cells, as these characteristics are relevant to cancer metastasis. METHODS: : Erlotinib-resistant PC-9ER cells were generated from PC-9 NSCLC cells, which harbor an EGFR-activating mutation, and used in this study. We investigated the involvement of the transforming growth factor beta (TGF-beta) pathway in cell motility, and tested the effects of erlotinib and TGF-beta type I receptor (RI) inhibition on cell motility. RESULTS: : PC-9ER cells displayed enhanced motility resulting from autocrine activation of the TGF-beta pathway. Increased TGF-beta2 secretion resulting from TGF-beta2 up-regulation at the transcriptional level was suggested to be responsible for the phosphorylation of Smad2 and the subsequently elevated transcriptional regulatory activity in PC-9ER cells. The motility of PC-9ER cells was suppressed by treatment with either the TGF-betaRI inhibitor LY364947 or erlotinib, and greater suppression was observed when used in combination. LY364947 or erlotinib exerted no growth-inhibitory effects, suggesting that motility and growth are driven by different signaling pathways in PC-9ER cells. CONCLUSIONS: : Our results imply that blockade of the TGF-beta signaling pathway combined with continuous EGFR TKI treatment will be beneficial in preventing metastasis in patients with EGFR TKI-resistant NSCLC without the EGFR T790M resistance mutation.
Dihydropyrrolopyrazole transforming growth factor-beta type I receptor kinase domain inhibitors: a novel benzimidazole series with selectivity versus transforming growth factor-beta type II receptor kinase and mixed lineage kinase-7.[Pubmed:16539403]
J Med Chem. 2006 Mar 23;49(6):2138-42.
Novel dihydropyrrolopyrazole-substituted benzimidazoles were synthesized and evaluated in vitro as inhibitors of transforming growth factor-beta type I receptor (TGF-beta RI), TGF-beta RII, and mixed lineage kinase-7 (MLK-7). These compounds were found to be potent TGF-beta RI inhibitors and selective versus TGF-beta RII and MLK-7 kinases. Benzimidazole derivative 8b was active in an in vivo target (TGF-beta RI) inhibition assay.
Smad4-dependent regulation of urokinase plasminogen activator secretion and RNA stability associated with invasiveness by autocrine and paracrine transforming growth factor-beta.[Pubmed:16959768]
J Biol Chem. 2006 Nov 10;281(45):33971-81.
Metastasis is a primary cause of mortality due to cancer. Early metastatic growth involves both a remodeling of the extracellular matrix surrounding tumors and invasion of tumors across the basement membrane. Up-regulation of extracellular matrix degrading proteases such as urokinase plasminogen activator (uPA) and matrix metalloproteinases has been reported to facilitate tumor cell invasion. Autocrine transforming growth factor-beta (TGF-beta) signaling may play an important role in cancer cell invasion and metastasis; however, the underlying mechanisms remain unclear. In the present study, we report that autocrine TGF-beta supports cancer cell invasion by maintaining uPA levels through protein secretion. Interestingly, treatment of paracrine/exogenous TGF-beta at higher concentrations than autocrine TGF-beta further enhanced uPA expression and cell invasion. The enhanced uPA expression by exogenous TGF-beta is a result of increased uPA mRNA expression due to RNA stabilization. We observed that both autocrine and paracrine TGF-beta-mediated regulation of uPA levels was lost upon depletion of Smad4 protein by RNA interference. Thus, through the Smad pathway, autocrine TGF-beta maintains uPA expression through facilitated protein secretion, thereby supporting tumor cell invasiveness, whereas exogenous TGF-beta further enhances uPA expression through mRNA stabilization leading to even greater invasiveness of the cancer cells.
Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain.[Pubmed:12954047]
J Med Chem. 2003 Sep 11;46(19):3953-6.
Pyrazole-based inhibitors of the transforming growth factor-beta type I receptor kinase domain (TbetaR-I) are described. Examination of the SAR in both enzyme- and cell-based in vitro assays resulted in the emergence of two subseries featuring differing selectivity versus p38 MAP kinase. A common binding mode at the active site has been established by successful cocrystallization and X-ray analysis of potent inhibitors with the TbetaR-I receptor kinase domain.


