SinigrinCAS# 3952-98-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3952-98-5 | SDF | Download SDF |
| PubChem ID | 23682211 | Appearance | White powder |
| Formula | C10H16KNO9S2 | M.Wt | 397.46 |
| Type of Compound | Glucosinolates | Storage | Desiccate at -20°C |
| Synonyms | Allylglucosinolate potassium salt; 2-Propenyl glucosinolate potassium salt;Allyl glucosinolate;534-69-0;64550-88-5 | ||
| Solubility | H2O : 125 mg/mL (314.50 mM; Need ultrasonic) | ||
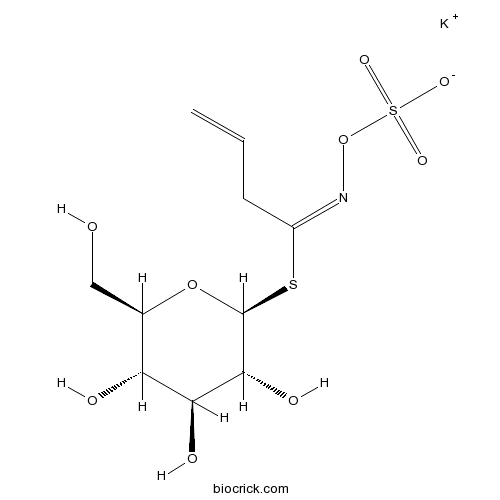
| Chemical Name | potassium;[(E)-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanylbut-3-enylideneamino] sulfate | ||
| SMILES | C=CCC(=NOS(=O)(=O)[O-])SC1C(C(C(C(O1)CO)O)O)O.[K+] | ||
| Standard InChIKey | QKFAFSGJTMHRRY-OCFLFPRFSA-M | ||
| Standard InChI | InChI=1S/C10H17NO9S2.K/c1-2-3-6(11-20-22(16,17)18)21-10-9(15)8(14)7(13)5(4-12)19-10;/h2,5,7-10,12-15H,1,3-4H2,(H,16,17,18);/q;+1/p-1/b11-6+;/t5-,7-,8+,9-,10+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sinigrin Dilution Calculator

Sinigrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.516 mL | 12.5799 mL | 25.1598 mL | 50.3195 mL | 62.8994 mL |
| 5 mM | 0.5032 mL | 2.516 mL | 5.032 mL | 10.0639 mL | 12.5799 mL |
| 10 mM | 0.2516 mL | 1.258 mL | 2.516 mL | 5.032 mL | 6.2899 mL |
| 50 mM | 0.0503 mL | 0.2516 mL | 0.5032 mL | 1.0064 mL | 1.258 mL |
| 100 mM | 0.0252 mL | 0.1258 mL | 0.2516 mL | 0.5032 mL | 0.629 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CC-401
Catalog No.:BCC4269
CAS No.:395104-30-0
- 3-Acetylcoumarin
Catalog No.:BCC8603
CAS No.:3949-36-8
- Boceprevir
Catalog No.:BCC1435
CAS No.:394730-60-0
- Eleutheroside E
Catalog No.:BCN1083
CAS No.:39432-56-9
- Ethyl 3,4-dihydroxybenzoate
Catalog No.:BCN8504
CAS No.:3943-89-3
- Kamebanin
Catalog No.:BCN5449
CAS No.:39388-57-3
- Neurotensin
Catalog No.:BCC5842
CAS No.:39379-15-2
- BCTC
Catalog No.:BCC7797
CAS No.:393514-24-4
- 2,3-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN6534
CAS No.:3934-81-4
- Tiplaxtinin(PAI-039)
Catalog No.:BCC6439
CAS No.:393105-53-8
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Ginsenoside Compound K
Catalog No.:BCN1246
CAS No.:39262-14-1
- Asperosaponin VI
Catalog No.:BCN1256
CAS No.:39524-08-8
- Nitrendipine
Catalog No.:BCC4381
CAS No.:39562-70-4
- Triamterene
Catalog No.:BCC5074
CAS No.:396-01-0
- Z-Sar-OH
Catalog No.:BCC3339
CAS No.:39608-31-6
- Pasireotide
Catalog No.:BCC5300
CAS No.:396091-73-9
- LY364947
Catalog No.:BCC5085
CAS No.:396129-53-6
- Isoshinanolone
Catalog No.:BCN7986
CAS No.:39626-91-0
- Azatadine
Catalog No.:BCC4133
CAS No.:3964-81-6
- BVT 948
Catalog No.:BCC2467
CAS No.:39674-97-0
- (R)-Reticuline
Catalog No.:BCN6795
CAS No.:3968-19-2
- 1,5-Diphenylpentan-1-one
Catalog No.:BCN7169
CAS No.:39686-51-6
- 2-Benzylsuccinic acid
Catalog No.:BCC8566
CAS No.:3972-36-9
The effect of processing on the glucosinolate profile in mustard seed.[Pubmed:29478552]
Food Chem. 2018 Jun 30;252:343-348.
Brassica juncea mustard seed are used to make mustard paste or condiment. Mustard seed contains glucosinolates which are converted to isothiocyanates following cell disruption by the enzyme, myrosinase. Isothiocyanates are sulphur-containing compounds which give a pungent flavour to the mustard condiment. Three mustard seed cultivars from two seasons were processed into Dijon- and wholegrain-style mustard and glucosinolates and isothiocyanates analysed. Canadian cv. Centennial tended to contain higher glucosinolates compared with the French cv. AZ147 and Ukrainian cv. Chorniava. Conversion of the mustard seed into a wholegrain condiment had a lesser effect on total isothiocyanates and Sinigrin content compared with the Dijon-style preparation. The Canadian mustard cultivars produced wholegrain-style mustard with higher total isothyocyantes and Sinigrin compared with the French and Ukrainian cultivars. In summary, results herein suggest that Canadian mustard seed cvs. Centennial and Forge, and wholegrain processing may result in a condiment with greater bioactive composition.
Inhibitory effect of sinigrin on adipocyte differentiation in 3T3-L1 cells: Involvement of AMPK and MAPK pathways.[Pubmed:29604586]
Biomed Pharmacother. 2018 Jun;102:670-680.
Adipocyte differentiation is a critical adaptive response to nutritional overload and affects the metabolic outcome of obesity. Sinigrin (2-propenyl glucosinolate) is a glucosinolate belong to the glucoside contained in broccoli, brussels sprouts, and black mustard seeds. We investigated the effects of Sinigrin on adipogenesis in 3T3-L1 preadipocytes and its underlying mechanisms. Sinigrin remarkably inhibited the accumulation of lipid droplets and adipogenesis by downregulating the expression of CCAAT-enhancer-binding protein alpha (C/EBPalpha), peroxisome proliferator-activated receptor gamma (PPARgamma), leptin and aP2. Sinigrin arrested cells in the G0/G1 phase of the cell cycle and increased the expression of p21 and p27. CDK2 expression was suppressed by sinigirn in MDI-induced adipocytes. Sinigrin increased the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK) and acetyl-CoA carboxylase (ACC) in the early stage of adipocyte differentiation, suggesting that Sinigrin has anti-adipogenic effects through AMPK, MAPK and ACC activation. Sinigrin also inhibited the production of pro-inflammatory cytokines including tumor necrosis factor -alpha (TNF-alpha) and interleukin (IL)-6, IL-1beta and IL-18. Taken together, these data suggest that Sinigrin inhibits early-stage adipogenesis of 3T3-L1 adipocytes through the AMPK and MAPK signaling pathways.
Effect of different proportion of sulphur treatments on the contents of glucosinolate in kale (Brassica oleracea var. acephala) commonly consumed in Republic of Korea.[Pubmed:29472789]
Saudi J Biol Sci. 2018 Feb;25(2):349-353.
Kale (Brassica oleracea L. Acephala Group) is the rich source of medicinal value sulphur compounds, glucosinolates (GLSs). The aim of this study was to investigate the effect of different proportion of sulphur (S) supplementation levels on the accumulation of GLSs in the leaves of the kale cultivar ('TBC'). High performance liquid chromatography (HPLC) separation method guided to identify and quantify six GSLs including three aliphatic (progoitrin, Sinigrin and gluconapin) and three indolyl (glucobrassicin, 4-methoxyglucobrassicin and neoglucobrasscin) respectively. Analysis of these distinct levels of S supplementation revealed that the accumulation of individual and total GLSs was directly proportional to the S concentration. The maximum levels of total GLSs (26.8 micromol/g DW) and glucobrassicin (9.98 micromol/g DW) were found in lower and upper parts of the leaves supplemented with 1 mM and 2 mM S, respectively. Interestingly, aliphatic GSLs were noted predominant in all the parts (50.1, 59.3 and 56% of total GSLs). Among the aliphatic and indolyl GSLs, Sinigrin and glucobrassicin account 35.3 and 30.88% of the total GSLs. From this study, it is concluded that supply of S enhance the GSLs accumulation in kale.
Allyl isothiocyanate reduces liver fibrosis by regulating Kupffer cell activation in rats.[Pubmed:29669958]
J Vet Med Sci. 2018 Jun 6;80(6):893-897.
Allyl isothiocyanate (AITC), a metabolite of the glucosinolate Sinigrin, protects the liver of rats injured by carbon tetrachloride (CCl4). This study evaluated whether AITC reduces hepatic fibrosis in rats repetitively exposed to CCl4. Serum chemistry showed that AITC (doses of 5 and 50 mg) administered to rats exposed to CCl4 significantly reduced the levels of alanine aminotransferase and aspartate aminotransferase activity that were elevated in CCl4-intoxicated rats. The connective tissue in AITC-treated rats was significantly reduced based on Sirius staining. In addition, Kupffer cell activation was significantly reduced in the AITC and CCl4 co-treated groups. Collectively, this study suggests that AITC mitigates hepatic fibrosis in rats repetitively exposed to CCl4 with concurrent regulation of Kupffer cell and monocyte activation.
Exposure of kale root to NaCl and Na2SeO3 increases isothiocyanate levels and Nrf2 signalling without reducing plant root growth.[Pubmed:29507323]
Sci Rep. 2018 Mar 5;8(1):3999.
A plant factory is a closed cultivation system that provides a consistent and modified environment for plant growth. We speculated that treatment of kale (Brassica oleracea) grown in a plant factory with NaCl, Na2SeO3, or both would increase the bioactive phytochemical levels including glucosinolates (GLSs) and isothiocyanates (ITCs), the key molecules in cancer prevention. The kale was harvested and analysed after treatment with NaCl and Na2SeO3 alone or in combination for 1 or 2 weeks. Exposure to NaCl alone but not Na2SeO3 increased plant root growth. Levels of Sinigrin were increased by a 2-week exposure to Na2SeO3 alone or in combination with NaCl, whereas no changes were observed in glucoraphanin and gluconasturtiin gluconasturtiin levels. Importantly, the ITC concentration was affected by 2-week treatment with both compounds. To evaluate the bioactivity of kale, HepG2 human hepatoma cells were treated with plant extract for 6 h. Only the extract of kale roots exposed to a combination NaCl and Na2SeO3 for 2 weeks showed an increased expression of nuclear factor erythroid 2-related factor (Nrf2), which regulates genes encoding antioxidant proteins. These data suggest that co-treatment with NaCl and Na2SeO3 increased the ITC content and chemopreventive effects of kale root.


