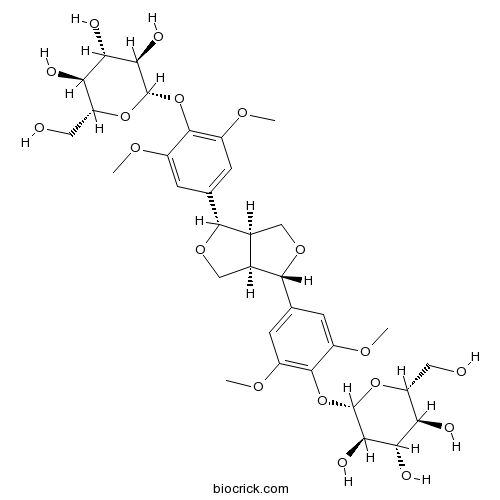
LiriodendrinCAS# 573-44-4 |
- Eleutheroside E
Catalog No.:BCN1083
CAS No.:39432-56-9
- Syringaresinol-di-O-glucoside
Catalog No.:BCN2600
CAS No.:66791-77-3
- Eleutheroside D
Catalog No.:BCN5336
CAS No.:79484-75-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 573-44-4 | SDF | Download SDF |
| PubChem ID | 21603207 | Appearance | Powder |
| Formula | C34H46O18 | M.Wt | 742.7 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[4-[(3S,3aR,6S,6aR)-6-[3,5-dimethoxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-3-yl]-2,6-dimethoxyphenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | COC1=CC(=CC(=C1OC2C(C(C(C(O2)CO)O)O)O)OC)C3C4COC(C4CO3)C5=CC(=C(C(=C5)OC)OC6C(C(C(C(O6)CO)O)O)O)OC | ||
| Standard InChIKey | FFDULTAFAQRACT-XKBSQSBASA-N | ||
| Standard InChI | InChI=1S/C34H46O18/c1-43-17-5-13(6-18(44-2)31(17)51-33-27(41)25(39)23(37)21(9-35)49-33)29-15-11-48-30(16(15)12-47-29)14-7-19(45-3)32(20(8-14)46-4)52-34-28(42)26(40)24(38)22(10-36)50-34/h5-8,15-16,21-30,33-42H,9-12H2,1-4H3/t15-,16-,21+,22+,23+,24+,25-,26-,27+,28+,29+,30+,33-,34-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Liriodendrin has anti-inflammatory and antinociceptive activities. 2. Liriodendrin shows an excellent hypoglycemic activity. 3. Liriodendrin has protective effects on dopamine-induced cytotoxicity via its anti-oxidative properties by reducing ROS level and anti-apoptotic effect via protection of mitochondrion membrane potential (ΔΨm). 4. Liriodendrin may be a potent suppressor of CaCl(2)-induced arrhythmias, the prophylactic administration of liriodendrin is effective in prolonging latency of arrhythmia and reducing the occurrence of ventricular fibrillation from 75% to 25%. 5. Liriodendrin has inhibitory activities on gastritis and gastric ulcer, it can inhibit colonization of Helicobacter pylori effectively, it could be utilized for the treatment and/or protection of gastritis and gastric ulcer. 6. Liriodendrin plays protective role in sepsis-induced acute lung injury, it regulates lung inflammation, the phosphorylation of the NF-kB (p65) and expression of vascular endothelial growth factor (VEGF). |
| Targets | PGE | ATPase | Potassium Channel | NO | TNF-α | NOS | COX | ROS | p53 | VEGFR | p65 | NF-kB |

Liriodendrin Dilution Calculator

Liriodendrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3464 mL | 6.7322 mL | 13.4644 mL | 26.9288 mL | 33.661 mL |
| 5 mM | 0.2693 mL | 1.3464 mL | 2.6929 mL | 5.3858 mL | 6.7322 mL |
| 10 mM | 0.1346 mL | 0.6732 mL | 1.3464 mL | 2.6929 mL | 3.3661 mL |
| 50 mM | 0.0269 mL | 0.1346 mL | 0.2693 mL | 0.5386 mL | 0.6732 mL |
| 100 mM | 0.0135 mL | 0.0673 mL | 0.1346 mL | 0.2693 mL | 0.3366 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boehmenan
Catalog No.:BCN5773
CAS No.:57296-22-7
- Ridaforolimus (Deforolimus, MK-8669)
Catalog No.:BCC4605
CAS No.:572924-54-0
- Boc-D-Phe(4-F)-OH
Catalog No.:BCC3218
CAS No.:57292-45-2
- Boc-D-Phe(4-Cl)-OH
Catalog No.:BCC3176
CAS No.:57292-44-1
- Calmidazolium chloride
Catalog No.:BCC7410
CAS No.:57265-65-3
- Setiptiline
Catalog No.:BCC1945
CAS No.:57262-94-9
- Salsolinol-1-carboxylic acid
Catalog No.:BCC6731
CAS No.:57256-34-5
- 7-Ethoxyresorufin
Catalog No.:BCC6476
CAS No.:5725-91-7
- Methoxyresorufin
Catalog No.:BCC6296
CAS No.:5725-89-3
- Pamidronate Disodium
Catalog No.:BCC1193
CAS No.:57248-88-1
- H-Phe(3-CN)-OH
Catalog No.:BCC3182
CAS No.:57213-48-6
- Testosterone decanoate
Catalog No.:BCC9168
CAS No.:5721-91-5
- Congo Red
Catalog No.:BCC8023
CAS No.:573-58-0
- Tacalcitol
Catalog No.:BCC1975
CAS No.:57333-96-7
- Dihydroepistephamiersine 6-acetate
Catalog No.:BCN5775
CAS No.:57361-74-7
- Bombinakinin-GAP
Catalog No.:BCC5903
CAS No.:573671-91-7
- Isochlorogenic acid C
Catalog No.:BCN2498
CAS No.:57378-72-0
- Irsogladine
Catalog No.:BCC4562
CAS No.:57381-26-7
- Oroxin A
Catalog No.:BCN1202
CAS No.:57396-78-8
- Benzoin ethyl ether
Catalog No.:BCC8855
CAS No.:574-09-4
- Isoflavone
Catalog No.:BCN8508
CAS No.:574-12-9
- Fraxetin
Catalog No.:BCN5903
CAS No.:574-84-5
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
- 8-O-Acetylshanzhiside methyl ester
Catalog No.:BCN5776
CAS No.:57420-46-9
Protective Effect of Liriodendrin Isolated from Kalopanax pictus against Gastric Injury.[Pubmed:25593644]
Biomol Ther (Seoul). 2015 Jan;23(1):53-9.
In this study, we investigated the inhibitory activities on gastritis and gastric ulcer using Liriodendrin which is a constituent isolated from Kalopanax pictus. To elucidate its abilities to prevent gastric injury, we measured the quantity of prostaglandin E2 (PGE2) as the protective factor, and we assessed inhibition of activities related to excessive gastric acid be notorious for aggressive factor and inhibition of Helicobacter pylori (H. pylori) colonization known as a cause of chronic gastritis, gastric ulcer, and gastric cancer. Liriodendrin exhibited higher PGE2 level than rebamipide used as a positive control group at the dose of 500 muM. It was also exhibited acid-neutralizing capacity (10.3%) and H(+)/K(+)-ATPase inhibition of 42.6% (500 muM). In pylorus-ligated rats, Liriodendrin showed lower volume of gastric juice (4.38 +/- 2.14 ml), slightly higher pH (1.53 +/- 0.41), and smaller total acid output (0.47 +/- 0.3 mEq/4 hrs) than the control group. Furthermore Liriodendrin inhibited colonization of H. pylori effectively. In vivo test, Liriodendrin significantly inhibited both of HCl/EtOH-induced gastritis (46.9 %) and indomethacin-induced gastric ulcer (46.1%). From these results, we suggest that Liriodendrin could be utilized for the treatment and/or protection of gastritis and gastric ulcer.
In vivo anti-inflammatory and antinociceptive effects of liriodendrin isolated from the stem bark of Acanthopanax senticosus.[Pubmed:12898415]
Planta Med. 2003 Jul;69(7):610-6.
In the present study, Liriodendrin isolated by activity-guided fractionation from the ethyl acetate (EtOAc) extracts of the stem bark of Acanthopanax senticosus, was evaluated for anti-inflammatory and antinociceptive activities. Liriodendrin (5, 10 mg/kg/day, p. o.) significantly inhibited the increase of vascular permeability induced by acetic acid in mice and reduced an acute paw edema induced by carrageenan in rats. When the analgesic activity was measured by the acetic acid-induced writhing test and hot plate test, Liriodendrin showed a dose-dependent inhibition in animal models. In addition, syringaresinol, the hydrolysate of Liriodendrin, more potently inhibited the LPS-induced production of NO, PGE 2 and TNF-alpha production of macrophages than Liriodendrin. Consistent with these observations, the expression level of iNOS and COX-2 enzyme was decreased by syringaresinol in a concentration-dependent manner. These results suggest that the anti-inflammatory and antinociceptive effects of Liriodendrin after oral administration were attributable to the in vivo transformation to syringaresinol, which may function as the active constituent.
Protective Role of Liriodendrin in Sepsis-Induced Acute Lung Injury.[Pubmed:27498121]
Inflammation. 2016 Oct;39(5):1805-13.
In current study, we investigated the role of Liriodendrin, a constituent isolated from Sargentodoxa cuneata (Oliv.) Rehd. Et Wils (Sargentodoxaceae), in cecal ligation and puncture (CLP)-induced acute lung inflammatory response and injury (ALI). The inflammatory mediator levels in bronchoalveolar lavage fluid (BALF) were determined by enzyme-linked immunosorbent assay (ELISA). Pathologic changes in lung tissues were evaluated via pathological section with hematoxylin and eosin (H&E) staining. To investigate the mechanism whereby Liriodendrin regulates lung inflammation, the phosphorylation of the NF-kB (p65) and expression of vascular endothelial growth factor (VEGF) were determined by western blot assay. We show that Liriodendrin treatment significantly improved the survival rate of mice with CLP-induced sepsis. Pulmonary histopathologic changes, alveolar hemorrhage, and neutrophil infiltration were markedly decreased by Liriodendrin. In addition, Liriodendrin decreased the production of the proinflammatory mediators including (TNF-alpha, IL-1beta, MCP-1, and IL-6) in lung tissues. Vascular permeability and lung myeloperoxidase (MPO) accumulation in the Liriodendrin-treated mice were substantially reduced. Moreover, Liriodendrin treatment significantly suppressed the expression of VEGF and activation of NF-kB in the lung. We further show that Liriodendrin significantly reduced the production of proinflammatory mediators and downregulated NF-kB signaling in LPS-stimulated RAW 264.7 macrophage cells. Moreover, Liriodendrin prevented the generation of reactive oxygen species (ROS) by upregulating the expression of SIRT1 in RAW 264.7 cells. These findings provide a novel theoretical basis for the possible application of Liriodendrin in clinic.
A new triterpene and an antiarrhythmic liriodendrin from Pittosporum brevicalyx.[Pubmed:21191756]
Arch Pharm Res. 2010 Dec;33(12):1927-32.
A new triterpene, 21-O-senecioyl-R(1)-barrigenol (1) and 13 known compounds were isolated from the ethanol extracts of the leaves and bark of Pittosporum brevicalyx (Oliv.) Gagnep. Their structures were elucidated based on spectral data. The antiarrhythmic action of one furofuran lignan, Liriodendrin (2), was tested on a model of CaCl(2)-induced arrhythmia and compared with the effect of verapamil. The prophylactic administration of Liriodendrin (2) was effective in prolonging latency of arrhythmia and reducing the occurrence of ventricular fibrillation from 75% to 25%. The overall mortality rate was significantly reduced by the prophylactic administration of Liriodendrin from 87.5% to 25%. The antiarrhythmic effect of Liriodendrin (5.0 mg/kg) was similar to that of verapamil (1.05 mg/kg). Thus, Liriodendrin may be a potent suppressor of CaCl(2)-induced arrhythmias.
Metabolism of liriodendrin and syringin by human intestinal bacteria and their relation to in vitro cytotoxicity.[Pubmed:10071956]
Arch Pharm Res. 1999 Feb;22(1):30-4.
When Liriodendrin or syringin was incubated for 24 h with human intestinal bacteria, two metabolites, (+)-syringaresinol-beta-D-glucopyranoside and (+)-syringaresinol, from Liriodendrin and one metabolite, synapyl alcohol, from syringin were produced. The metabolic time course of Liriodendrin was as follows: at early time, Liriodendrin was converted to (+)-syringaresinol-beta-D-glucopyranoside, and then (+)-syringaresinol. The in vitro cytotoxicities of these metabolites, (+)-syringaresinol and synapyl alcohol, were superior to those of Liriodendrin and syringin.


