FraxetinCAS# 574-84-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 574-84-5 | SDF | Download SDF |
| PubChem ID | 5273569 | Appearance | White powder |
| Formula | C10H8O5 | M.Wt | 208.17 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | 7,8-Dihydroxy 6-methoxycoumarin; Fratexin; Fraxetol | ||
| Solubility | DMSO : 250 mg/mL (1200.94 mM; Need ultrasonic) | ||
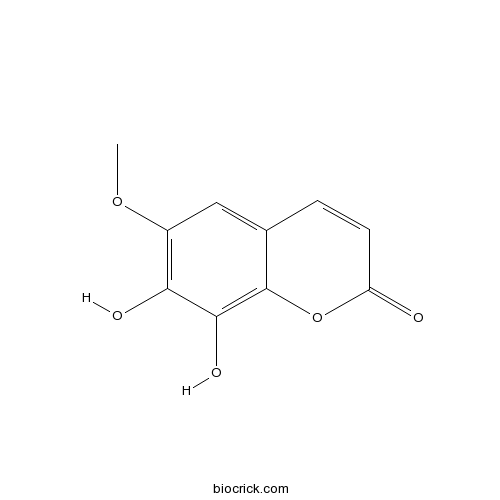
| Chemical Name | 7,8-dihydroxy-6-methoxychromen-2-one | ||
| SMILES | COC1=C(C(=C2C(=C1)C=CC(=O)O2)O)O | ||
| Standard InChIKey | HAVWRBANWNTOJX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H8O5/c1-14-6-4-5-2-3-7(11)15-10(5)9(13)8(6)12/h2-4,12-13H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fraxetin has dual-antioxidative ,hepatoprotective and antihyperglycemic functions, it shows potent protective effects against CCl4 induced oxidative stress and hepatic fibrosis, has a marked inhibitory effect on S.aureus proliferation. It increased the level of Nrf2/ARE, and HO-1, inhibit the formation of ROS, cytochrome c release, activation of caspase-3 and 9, and suppressed the up-regulation of Bax. |
| Targets | Nrf2 | LDL | ROS | Caspase |
| In vitro | Antibacterial mechanism of fraxetin against Staphylococcus aureus.[Pubmed: 25189268]Mol Med Rep. 2014 Nov;10(5):2341-5.Fraxetin is one of the main constituents of the traditional medicinal plant Fraxinus rhynchophylla. The inhibitory effect of Fraxetin on various bacterial strains has been extensively reported, however, its mechanism of action on bacterial cells remains to be elucidated.
Fraxetin prevents rotenone-induced apoptosis by induction of endogenous glutathione in human neuroblastoma cells.[Pubmed: 15996779 ]Neurosci Res. 2005 Sep;53(1):48-56.Fraxetin belongs to an extensive group of natural phenolic anti-oxidants.
|
| In vivo | Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives.[Pubmed: 24176844]Phytomedicine. 2014 Feb 15;21(3):240-6.Coumarins, also known as benzopyrones, are plant-derived products with several pharmacological properties, including antioxidant and anti-inflammatory activities. Based on the wide distribution of coumarin derivatives in plant-based foods and beverages in the human diet, our objective was to evaluate both the antioxidant and intestinal anti-inflammatory activities of six coumarin derivatives of plant origin (scopoletin, scoparone, Fraxetin, 4-methyl-umbeliferone, esculin and daphnetin) to verify if potential intestinal anti-inflammatory activity was related to antioxidant properties.
Antihyperglycemic effect of fraxetin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats.[Pubmed: 23806420]Biochimie. 2013 Oct;95(10):1848-54.Epidemiological studies have demonstrated that the diabetes mellitus is a serious health burden for both governments and healthcare providers.
|
| Cell Research | Dual anti-oxidative effects of fraxetin isolated from Fraxinus rhinchophylla.[Pubmed: 19721227]Biol Pharm Bull. 2009 Sep;32(9):1527-32.Atherosclerosis is main cause of arteriosclerosis. The pivotal role of low-density lipoprotein (LDL) oxidation in atherogenesis suggests antioxidants may help prevent cardiovascular disease. Fraxinus rhynchophylla DENCE (Oleaceae) is a traditional medicinal plant from East Asia.
|
| Animal Research | The hepatoprotective effect of fraxetin on carbon tetrachloride induced hepatic fibrosis by antioxidative activities in rats.[Pubmed: 23994349 ]Int Immunopharmacol. 2013 Nov;17(3):543-7.The aim of the study was to investigate the potentially protective effects of Fraxetin on carbon tetrachloride (CCl4) induced oxidative stress and hepatic fibrosis in Sprague-Dawley rats.
|

Fraxetin Dilution Calculator

Fraxetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8038 mL | 24.0188 mL | 48.0377 mL | 96.0753 mL | 120.0942 mL |
| 5 mM | 0.9608 mL | 4.8038 mL | 9.6075 mL | 19.2151 mL | 24.0188 mL |
| 10 mM | 0.4804 mL | 2.4019 mL | 4.8038 mL | 9.6075 mL | 12.0094 mL |
| 50 mM | 0.0961 mL | 0.4804 mL | 0.9608 mL | 1.9215 mL | 2.4019 mL |
| 100 mM | 0.048 mL | 0.2402 mL | 0.4804 mL | 0.9608 mL | 1.2009 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isoflavone
Catalog No.:BCN8508
CAS No.:574-12-9
- Benzoin ethyl ether
Catalog No.:BCC8855
CAS No.:574-09-4
- Oroxin A
Catalog No.:BCN1202
CAS No.:57396-78-8
- Irsogladine
Catalog No.:BCC4562
CAS No.:57381-26-7
- Isochlorogenic acid C
Catalog No.:BCN2498
CAS No.:57378-72-0
- Bombinakinin-GAP
Catalog No.:BCC5903
CAS No.:573671-91-7
- Dihydroepistephamiersine 6-acetate
Catalog No.:BCN5775
CAS No.:57361-74-7
- Tacalcitol
Catalog No.:BCC1975
CAS No.:57333-96-7
- Congo Red
Catalog No.:BCC8023
CAS No.:573-58-0
- Liriodendrin
Catalog No.:BCN5774
CAS No.:573-44-4
- Boehmenan
Catalog No.:BCN5773
CAS No.:57296-22-7
- Ridaforolimus (Deforolimus, MK-8669)
Catalog No.:BCC4605
CAS No.:572924-54-0
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
- 8-O-Acetylshanzhiside methyl ester
Catalog No.:BCN5776
CAS No.:57420-46-9
- (2S)-2alpha-(1,3-Benzodioxol-5-yl)-3,5-dihydro-5alpha-methoxy-3beta-methyl-5-allyl-2H-benzofuran-6-one
Catalog No.:BCN6606
CAS No.:57430-03-2
- Methylergometrine maleate
Catalog No.:BCC6691
CAS No.:57432-61-8
- Resiniferatoxin
Catalog No.:BCC6951
CAS No.:57444-62-9
- p-Menth-8-ene-1,2-diol
Catalog No.:BCN5777
CAS No.:57457-97-3
- 2-Epi-3a-epiburchellin
Catalog No.:BCN7013
CAS No.:57457-99-5
- Stigmasta-3,5-diene
Catalog No.:BCC8254
CAS No.:4970-37-0
- H-Asn-OMe.HCl
Catalog No.:BCC2876
CAS No.:57461-34-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
- 16-Deoxysaikogenin F
Catalog No.:BCN6414
CAS No.:57475-62-4
- BRL-54443
Catalog No.:BCC5047
CAS No.:57477-39-1
Antihyperglycemic effect of fraxetin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats.[Pubmed:23806420]
Biochimie. 2013 Oct;95(10):1848-54.
Epidemiological studies have demonstrated that the diabetes mellitus is a serious health burden for both governments and healthcare providers. The present study was hypothesized to evaluate the antihyperglycemic potential of Fraxetin by determining the activities of key enzymes of carbohydrate metabolism in streptozotocin (STZ) - induced diabetic rats. Diabetes was induced in male albino Wistar rats by intraperitoneal administration of STZ (40 mg/kg b.w). Fraxetin was administered to diabetic rats intra gastrically at 20, 40, 80 mg/kg b.w for 30 days. The dose 80 mg/kg b.w, significantly reduced the levels of blood glucose and glycosylated hemoglobin (HbA1c) and increased plasma insulin level. The altered activities of the key enzymes of carbohydrate metabolism such as glucokinase, glucose-6-phosphate dehydrogenase, glucose-6-phosphatase, fructose-1,6-bisphosphatase and hepatic enzymes (aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP)) in the liver tissues of diabetic rats were significantly reverted to near normal levels by the administration of Fraxetin. Further, Fraxetin administration to diabetic rats improved body weight and hepatic glycogen content demonstrated its antihyperglycemic potential. The present findings suggest that Fraxetin may be useful in the treatment of diabetes even though clinical studies to evaluate this possibility may be warranted.
The hepatoprotective effect of fraxetin on carbon tetrachloride induced hepatic fibrosis by antioxidative activities in rats.[Pubmed:23994349]
Int Immunopharmacol. 2013 Nov;17(3):543-7.
The aim of the study was to investigate the potentially protective effects of Fraxetin on carbon tetrachloride (CCl4) induced oxidative stress and hepatic fibrosis in Sprague-Dawley rats. In this study, rats were divided into five groups, including normal controls, model, silymarin as the positive control, Fraxetin 20 mg/kg and Fraxetin 50 mg/kg. After 8 weeks, activities of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) were checked. The levels of protein carbonyls, thiobarbituric acid-reactive substances (TBARS) and antioxidant enzymes such as catalase, SOD and glutathione peroxidase (GSH-Px) were determined after Fraxetin administration. The hydroxyproline levels and histopathologic examinations of hepatocyte fibrosis were also determined. We found that Fraxetin at doses of 20 and 50 mg/kg for 8 weeks significantly reduced the levels of TBARS and protein carbonyls compared with CCl4 group. Fraxetin significantly increased the activities of catalase, SOD and GSH-Px in the liver. We also found that Fraxetin prevented CCl4 induced hepatic fibrosis by histological observations. These results indicate that Fraxetin exhibits potent protective effects against CCl4 induced oxidative stress and hepatic fibrosis.
Antibacterial mechanism of fraxetin against Staphylococcus aureus.[Pubmed:25189268]
Mol Med Rep. 2014 Nov;10(5):2341-5.
Fraxetin is one of the main constituents of the traditional medicinal plant Fraxinus rhynchophylla. The inhibitory effect of Fraxetin on various bacterial strains has been extensively reported, however, its mechanism of action on bacterial cells remains to be elucidated. In the present study, the antibacterial mechanism of Fraxetin on Staphylococcus aureus was systematically investigated by examining its effect on cell membranes, protein synthesis, nucleic acid content and topoisomerase activity. The results indicated that Fraxetin increased the permeability of the cell membrane but did not render it permeable to macromolecules, such as DNA and RNA. Additionally, the quantity of protein, DNA and RNA decreased to 55.74, 33.86 and 48.96%, respectively following treatment with Fraxetin for 16 h. The activity of topoisomerase I and topoisomerase II were also markedly inhibited as Fraxetin concentration increased. The result of the ultravioletvisible spectrophotometry demonstrated that the DNA characteristics exhibited a blue shift and hypochromic effect following treatment with Fraxetin. These results indicated that Fraxetin had a marked inhibitory effect on S.aureus proliferation. Further mechanistic studies showed that Fraxetin could disrupt nucleic acid and protein synthesis by preventing topoisomerase from binding to DNA.
Dual anti-oxidative effects of fraxetin isolated from Fraxinus rhinchophylla.[Pubmed:19721227]
Biol Pharm Bull. 2009 Sep;32(9):1527-32.
Atherosclerosis is main cause of arteriosclerosis. The pivotal role of low-density lipoprotein (LDL) oxidation in atherogenesis suggests antioxidants may help prevent cardiovascular disease. Fraxinus rhynchophylla DENCE (Oleaceae) is a traditional medicinal plant from East Asia. During the course of characterizing potential drug candidates from natural products, we isolated two major coumarins, esculetin and Fraxetin and found that Fraxetin has dual-antioxidative functions. Low concentrations (1-5 microM) of Fraxetin potently inhibited LDL oxidation induced by metal and free radicals. Moreover, treatment of vascular smooth muscle cells (VSMCs) with higher concentrations (above 30 microM) of Fraxetin significantly increased the protein level of heme oxygenase-1 (HO-1), a key enzyme that inhibits vascular proliferation and atherosclerosis. Subcellular fractionation and reporter gene analysis using an antioxidant response element (ARE) construct revealed that Fraxetin increased the level of nuclear factor (NF)-E2-related factor 2 (Nrf2) and reporter activity, and these were associated with the induction of antioxidant enzymes, such as HO-1 and glutathione S-transferase-alpha. In conclusion, Fraxetin has direct protective properties against LDL oxidation at lower concentrations, and higher concentrations of Fraxetin induce antioxidant enzymes via Nrf2/ARE activation. These effects suggest potential anti-atherosclerosis effects of Fraxinus rhynchophylla D.
Fraxetin prevents rotenone-induced apoptosis by induction of endogenous glutathione in human neuroblastoma cells.[Pubmed:15996779]
Neurosci Res. 2005 Sep;53(1):48-56.
Fraxetin belongs to an extensive group of natural phenolic anti-oxidants. In the present study, using a human neuroblastoma SH-SY5Y cells, we have investigated the protective effects of this compound on modifications in endogenous reduced glutathione (GSH), intracellular oxygen species (ROS) and apoptotic death on rotenone-mediated cytoxicity. Incubation of cells with the Fraxetin led to a significant elevation dose-dependent of cellular GSH and this was accompanied by a marked protection against rotenone-mediated toxicity, which was also significantly reversed in the cells with buthionine sulfoximine (BSO) co-treatment. Taken together, this study suggested that intracellular GSH appeared to be an important factor in Fraxetin-mediated cytoprotection against rotenone-toxicity in SH-SY5Y cells. Fraxetin at 10-100 muM inhibited the formation of ROS, cytochrome c release, activation of caspase-3 and 9, and suppressed the up-regulation of Bax, whereas no significant change occurred in Bcl-2 levels. Our results indicated that the anti-oxidative and anti-apoptotic properties render this natural compound potentially protective against rotenone-induced cytotoxicity.
Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives.[Pubmed:24176844]
Phytomedicine. 2014 Feb 15;21(3):240-6.
BACKGROUND: Coumarins, also known as benzopyrones, are plant-derived products with several pharmacological properties, including antioxidant and anti-inflammatory activities. Based on the wide distribution of coumarin derivatives in plant-based foods and beverages in the human diet, our objective was to evaluate both the antioxidant and intestinal anti-inflammatory activities of six coumarin derivatives of plant origin (scopoletin, scoparone, Fraxetin, 4-methyl-umbeliferone, esculin and daphnetin) to verify if potential intestinal anti-inflammatory activity was related to antioxidant properties. METHODS: Intestinal inflammation was induced by intracolonic instillation of TNBS in rats. The animals were treated with coumarins by oral route. The animals were killed 48 h after colitis induction. The colonic segments were obtained after laparotomy and macroscopic and biochemical parameters (determination of glutathione level and myeloperoxidase and alkaline phosphatase activities) were evaluated. The antioxidant properties of these coumarins were examined by lipid peroxidation and DPPH assays. RESULTS: Treatment with esculin, scoparone and daphnetin produced the best protective effects. All coumarin derivatives showed antioxidant activity in the DPPH assay, while daphnetin and Fraxetin also showed antioxidant activity by inhibiting lipid peroxidation. Coumarins, except 4-methyl-umbeliferone, also showed antioxidant activity through the counteraction of glutathione levels or through the inhibition of myeloperoxidase activity. DISCUSSION: The intestinal anti-inflammatory activity of coumarin derivatives were related to their antioxidant properties, suggesting that consumption of coumarins and/or foods rich in coumarin derivatives, particularly daphnetin, esculin and scoparone, could prevent intestinal inflammatory disease.


