PD184352 (CI-1040)Selective MEK inhibitor CAS# 212631-79-3 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 212631-79-3 | SDF | Download SDF |
| PubChem ID | 6918454 | Appearance | Powder |
| Formula | C17H14ClF2IN2O2 | M.Wt | 478.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CI-1040 | ||
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol | ||
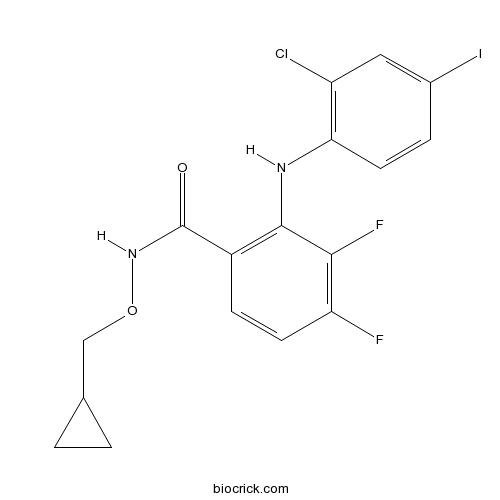
| Chemical Name | 2-(2-chloro-4-iodoanilino)-N-(cyclopropylmethoxy)-3,4-difluorobenzamide | ||
| SMILES | C1CC1CONC(=O)C2=C(C(=C(C=C2)F)F)NC3=C(C=C(C=C3)I)Cl | ||
| Standard InChIKey | GFMMXOIFOQCCGU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14ClF2IN2O2/c18-12-7-10(21)3-6-14(12)22-16-11(4-5-13(19)15(16)20)17(24)23-25-8-9-1-2-9/h3-7,9,22H,1-2,8H2,(H,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective MEK inhibitor (Ki = 300 nM in vitro). Suppresses FGF-mediated angiogenesis in vivo and decreases VEGF expression. Enhances the therapeutic efficacy of taxol in vivo. Orally active. |

PD184352 (CI-1040) Dilution Calculator

PD184352 (CI-1040) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0891 mL | 10.4456 mL | 20.8912 mL | 41.7824 mL | 52.228 mL |
| 5 mM | 0.4178 mL | 2.0891 mL | 4.1782 mL | 8.3565 mL | 10.4456 mL |
| 10 mM | 0.2089 mL | 1.0446 mL | 2.0891 mL | 4.1782 mL | 5.2228 mL |
| 50 mM | 0.0418 mL | 0.2089 mL | 0.4178 mL | 0.8356 mL | 1.0446 mL |
| 100 mM | 0.0209 mL | 0.1045 mL | 0.2089 mL | 0.4178 mL | 0.5223 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PD184352 (also known as CI-1040), a benzhydroxamate derivative, is a potent and highly selective MEK1/2, two members of the family of MAPKKs, inhibitor that inhibits purified MEK1 with IC50 of 17 nM in a non-ATP and non-ERK1/2 competitive manner [1].
PD184352 binds to a hydrophobic pocket, which is located in a region with low sequence homology to other kinases, adjacent to the Mg-ATP binding site of MEK1 and MEK2 inducing a conformational change in un-phosphorylated MEK1/2 and hence inactivating the un-phosphorylated MEK1/2 [1].
PD184352 has been found to be actively against tumors, where it inhibits the growth of colon carcinomas in mouse xenograft models [1].
References:
[1] Frémin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol. 2010 Feb 11;3:8. doi: 10.1186/1756-8722-3-8.
- PD 198306
Catalog No.:BCC7428
CAS No.:212631-61-3
- Nocistatin (human)
Catalog No.:BCC5732
CAS No.:212609-11-5
- Ebracteolatanolide A
Catalog No.:BCN3773
CAS No.:212563-72-9
- Oxaprozin
Catalog No.:BCC9109
CAS No.:21256-18-8
- HS 024
Catalog No.:BCC5820
CAS No.:212370-59-7
- TC 2559 difumarate
Catalog No.:BCC7469
CAS No.:212332-35-9
- Ethyl 3-(3-amino-4-(methylamino)-N-(pyridin-2-yl)benzamido)propanoate
Catalog No.:BCC8971
CAS No.:212322-56-0
- Ipfencarbazone
Catalog No.:BCC5465
CAS No.:212201-70-2
- 5,7-Dihydroxy-3-(4-hydroxy-3,5-dimethoxybenzyl)-6,8-dimethylchroman-4-one
Catalog No.:BCN6631
CAS No.:212201-12-2
- Apparicine
Catalog No.:BCN4008
CAS No.:2122-36-3
- Vatalanib
Catalog No.:BCC2085
CAS No.:212141-54-3
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Xanthiazone
Catalog No.:BCN2544
CAS No.:212701-97-8
- NG 52
Catalog No.:BCC1798
CAS No.:212779-48-1
- Ramage Linker,Fmoc-Suberol
Catalog No.:BCC2834
CAS No.:212783-75-0
- Kuramerine
Catalog No.:BCN1806
CAS No.:21284-19-5
- Kumokirine
Catalog No.:BCN2011
CAS No.:21284-20-8
- Cowaxanthone B
Catalog No.:BCN3892
CAS No.:212842-64-3
- Purvalanol A
Catalog No.:BCC5654
CAS No.:212844-53-6
- Purvalanol B
Catalog No.:BCC3887
CAS No.:212844-54-7
- (S)-(+)-Abscisic acid
Catalog No.:BCN2210
CAS No.:21293-29-8
- Tetrahymanol
Catalog No.:BCN6934
CAS No.:2130-17-8
- Tetrahymanol acetate
Catalog No.:BCN6933
CAS No.:2130-22-5
- Boc-Tyr(Bzl)-OH
Catalog No.:BCC3461
CAS No.:2130-96-3
Central role of Fas-associated death domain protein in apoptosis induction by the mitogen-activated protein kinase kinase inhibitor CI-1040 (PD184352) in acute lymphocytic leukemia cells in vitro.[Pubmed:12963734]
J Biol Chem. 2003 Nov 21;278(47):47326-39.
Because the MAPK pathway plays important roles in cell proliferation and inhibition of apoptosis, this pathway has emerged as a potential therapeutic target for solid tumors and leukemia. At the present time there is little information about activation of this pathway and the consequences of its inhibition in acute lymphocytic leukemia cells (ALL). In the present study, constitutive MAPK pathway activation, as evidenced by phosphorylation of ERK1 and ERK2, was observed in 8 of 8 human lymphoid cell lines and 33% (8:24) of pretreatment ALL bone marrows. Inhibition of this pathway by the MEK inhibitors CI-1040 and PD098059 induced apoptosis through a unique pathway involving dephosphorylation and aggregation of Fas-associated death domain protein followed by death receptor-independent caspase-8 activation. Jurkat cell variants lacking Fas-associated death domain protein or procaspase-8 were resistant to CI-1040-induced apoptosis, as were Jurkat or Molt3 cells treated with the O-methyl ester of the caspase-8 inhibitor N-(Nalpha-benzyloxycarbonylisoleucylglutamyl) aspartate fluoromethyl ketone. In contrast, CI-1040-induced apoptosis was unaffected by blocking anti-Fas antibody, soluble tumor necrosis factor-alpha-related apoptosis-inducing ligand decoy receptor, or transfection with cDNA encoding the anti-apoptotic Bcl-2 family member Mcl-1 or dominant negative caspase-9. Collectively, these results identify the MAPK pathway as a potential therapeutic target in ALL and delineate a mechanism by which MEK inhibition triggers apoptosis in ALL cells.
CI-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK).[Pubmed:14613031]
Semin Oncol. 2003 Oct;30(5 Suppl 16):105-16.
Several key growth factors, cytokines, and proto-oncogenes transduce their growth- and differentiation-promoting signals through the mitogen-activated protein kinase or extracellular signal-regulated protein kinase (ERK) cascade. Overexpression or constitutive activation of this pathway has been shown to play an important role in the pathogenesis and progression of breast and other cancers, making the components of this signaling cascade potentially important as therapeutic targets. CI-1040 (PD184352) is an orally active, highly specific, small-molecule inhibitor of one of the key components of this pathway (MEK1/MEK2), and thereby effectively blocks the phosphorylation of ERK and continued signal transduction through this pathway. Antitumor activity has been seen in preclinical models with this compound, particularly for pancreas, colon, and breast cancers, which has been shown to correlate with its inhibition of pERK. Clinically, CI-1040 has been shown to be well tolerated in phase I studies, with safety and pharmacokinetic profiles that permit continuous daily dosing. Biomarker studies have shown target inhibition in patients, and antitumor activity has also been observed with a partial response in one patient with pancreatic cancer and stable disease in approximately 25% of phase I patients. Given the central role of the ERK/mitogen-activated protein kinase pathway in mediating growth-promoting signals for a diverse group of upstream stimuli, inhibitors of MEK, as a key central mediator, could have significant clinical benefit in the treatment of breast and other cancers.
The mitogen-activated protein kinase/extracellular signal-regulated kinase kinase inhibitor PD184352 (CI-1040) selectively induces apoptosis in malignant schwannoma cell lines.[Pubmed:16239399]
J Pharmacol Exp Ther. 2006 Jan;316(1):456-65.
Type 1 neurofibromatosis (NF1) is a common autosomal dominant disorder that results in neuroectodermal tumors. The NF1 tumor-suppressor gene encodes neurofibromin, which includes a GTPase-activating domain for Ras inactivation. Affinity purification showed N-Ras to be the predominant activated isoform of Ras in two independent neurofibrosarcoma cell lines from NF1 patients (lines ST88-14 and NF90-8). These NF1 cells also demonstrated increased constitutive activity of the extracellular signal-regulated kinases 1 and 2 (ERK1,2) mitogen-activated protein (MAP) kinases compared with a sporadic malignant schwannoma cell line that maintains neurofibromin expression (STS-26T). Thus, MAP kinase kinase (MEK) inhibitors may be a rational approach to NF1 therapy. The MEK inhibitors PD98059 [2'-amino-3'-methoxyflavone], PD184352 (also called CI-1040) [2-(2-chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-benzamide], and U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene] all produced concentration-dependent suppression of the proliferation of the three cell lines. Individual MEK inhibitors had similar effects in all three cell lines. However, only the antiproliferative effects of PD184352 correlated closely with the elimination of ERK1,2 MAP kinase activities. PD98059 was primarily cytostatic, whereas U0126 and PD184352 were cytotoxic. Only PD184352 induced apoptosis in all three lines, as indicated by morphology, activation of DEVDase, procaspase-3 cleavage, and the appearance of populations having sub-G(0)/G(1) DNA contents. The differential effects of the MEK inhibitors on cell survival were not dependent on p53 status or effects on the ERK5 pathway. PD184352 was also proapoptotic to primary rat Schwann cells. Hence, although PD184352 effectively killed neurofibrosarcoma cells, its effects on normal Schwann cells may limit its usefulness in the clinic.
BRAF mutation predicts sensitivity to MEK inhibition.[Pubmed:16273091]
Nature. 2006 Jan 19;439(7074):358-62.
The kinase pathway comprising RAS, RAF, mitogen-activated protein kinase kinase (MEK) and extracellular signal regulated kinase (ERK) is activated in most human tumours, often through gain-of-function mutations of RAS and RAF family members. Using small-molecule inhibitors of MEK and an integrated genetic and pharmacologic analysis, we find that mutation of BRAF is associated with enhanced and selective sensitivity to MEK inhibition when compared to either 'wild-type' cells or cells harbouring a RAS mutation. This MEK dependency was observed in BRAF mutant cells regardless of tissue lineage, and correlated with both downregulation of cyclin D1 protein expression and the induction of G1 arrest. Pharmacological MEK inhibition completely abrogated tumour growth in BRAF mutant xenografts, whereas RAS mutant tumours were only partially inhibited. These data suggest an exquisite dependency on MEK activity in BRAF mutant tumours, and offer a rational therapeutic strategy for this genetically defined tumour subtype.
Enhancement of the therapeutic efficacy of taxol by the mitogen-activated protein kinase kinase inhibitor CI-1040 in nude mice bearing human heterotransplants.[Pubmed:15805287]
Cancer Res. 2005 Apr 1;65(7):2854-60.
Taxol may contribute to intrinsic chemoresistance by activating the mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) cytoprotective pathway in human cancer cell lines and tumors. We have previously shown additivity between Taxol and the MEK inhibitor, U0126 in human cancer cell lines. Here, the combination of Taxol with an orally bioavailable MEK inhibitor, CI-1040, was evaluated in human lung tumors heterotransplanted into nude mice. Unlike xenograft models that are derived from cells with multiple genetic alterations due to prolonged passage, heterotransplanted tumor models are more clinically relevant. Combined treatment with both drugs resulted in inhibition of tumor growth in all models and tumor regressions in three of four models tested, supporting our previous observation that Taxol's efficacy is potentiated by MEK inhibition. Concurrent administration was superior to intermittent dosing. Pharmacodynamic assessments of tumors indicated that suppression of MEK was associated with induction of S473 phosphorylated Akt and reduced proliferation in the combination groups relative to single agents, in addition to suppression of fibroblast growth factor-mediated angiogenesis and reduced expression of vascular endothelial growth factor. These findings are significant and indicate that this combination may have broad therapeutic applications in a diverse range of lung tumors with different intrinsic chemosensitivities.


