Purvalanol ACyclin-dependent kinase inhibitor CAS# 212844-53-6 |
- THZ1
Catalog No.:BCC4005
CAS No.:1604810-83-4
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AZD-5438
Catalog No.:BCC3689
CAS No.:602306-29-6
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 212844-53-6 | SDF | Download SDF |
| PubChem ID | 456214 | Appearance | Powder |
| Formula | C19H25ClN6O | M.Wt | 388.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NG 60 | ||
| Solubility | DMSO : ≥ 50 mg/mL (128.57 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
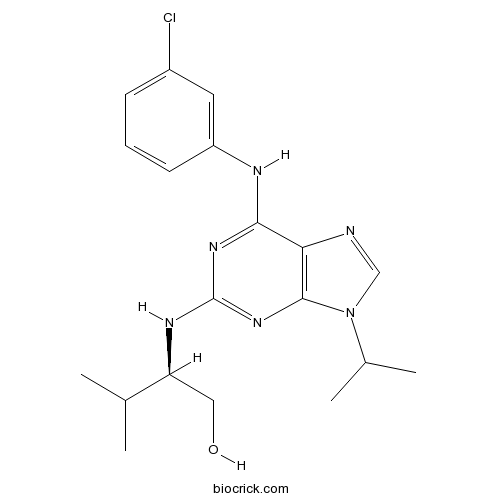
| Chemical Name | (2R)-2-[[6-(3-chloroanilino)-9-propan-2-ylpurin-2-yl]amino]-3-methylbutan-1-ol | ||
| SMILES | CC(C)C(CO)NC1=NC2=C(C(=N1)NC3=CC(=CC=C3)Cl)N=CN2C(C)C | ||
| Standard InChIKey | PMXCMJLOPOFPBT-HNNXBMFYSA-N | ||
| Standard InChI | InChI=1S/C19H25ClN6O/c1-11(2)15(9-27)23-19-24-17(22-14-7-5-6-13(20)8-14)16-18(25-19)26(10-21-16)12(3)4/h5-8,10-12,15,27H,9H2,1-4H3,(H2,22,23,24,25)/t15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclin-dependent kinase inhibitor. IC50 values are 4, 70, 35, 850 and 75 nM for cdc2/cyclin B, cdk2/cyclin A, cdk2/cyclin E, cdk4/cyclin D1 and cdk5-p35 respectively. Reversibly arrests synchronised cells in G1 and G2, and inhibits cell proliferation and cell death. |

Purvalanol A Dilution Calculator

Purvalanol A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5714 mL | 12.8571 mL | 25.7142 mL | 51.4284 mL | 64.2855 mL |
| 5 mM | 0.5143 mL | 2.5714 mL | 5.1428 mL | 10.2857 mL | 12.8571 mL |
| 10 mM | 0.2571 mL | 1.2857 mL | 2.5714 mL | 5.1428 mL | 6.4286 mL |
| 50 mM | 0.0514 mL | 0.2571 mL | 0.5143 mL | 1.0286 mL | 1.2857 mL |
| 100 mM | 0.0257 mL | 0.1286 mL | 0.2571 mL | 0.5143 mL | 0.6429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Purvalanol A is a potent, and cell-permeable CDK inhibitor with IC50 of 4 nM, 70 nM, 35 nM, and 850 nM for cdc2-cyclin B, cdk2-cyclin A, cdk2-cyclin E, and cdk4-cyclin D1, respectively.
- Cowaxanthone B
Catalog No.:BCN3892
CAS No.:212842-64-3
- Kumokirine
Catalog No.:BCN2011
CAS No.:21284-20-8
- Kuramerine
Catalog No.:BCN1806
CAS No.:21284-19-5
- Ramage Linker,Fmoc-Suberol
Catalog No.:BCC2834
CAS No.:212783-75-0
- NG 52
Catalog No.:BCC1798
CAS No.:212779-48-1
- Xanthiazone
Catalog No.:BCN2544
CAS No.:212701-97-8
- PD184352 (CI-1040)
Catalog No.:BCC1112
CAS No.:212631-79-3
- PD 198306
Catalog No.:BCC7428
CAS No.:212631-61-3
- Nocistatin (human)
Catalog No.:BCC5732
CAS No.:212609-11-5
- Ebracteolatanolide A
Catalog No.:BCN3773
CAS No.:212563-72-9
- Oxaprozin
Catalog No.:BCC9109
CAS No.:21256-18-8
- HS 024
Catalog No.:BCC5820
CAS No.:212370-59-7
- Purvalanol B
Catalog No.:BCC3887
CAS No.:212844-54-7
- (S)-(+)-Abscisic acid
Catalog No.:BCN2210
CAS No.:21293-29-8
- Tetrahymanol
Catalog No.:BCN6934
CAS No.:2130-17-8
- Tetrahymanol acetate
Catalog No.:BCN6933
CAS No.:2130-22-5
- Boc-Tyr(Bzl)-OH
Catalog No.:BCC3461
CAS No.:2130-96-3
- Org 37684
Catalog No.:BCC6291
CAS No.:213007-95-5
- Ceanothic acid
Catalog No.:BCN4918
CAS No.:21302-79-4
- [Phe1Ψ(CH2-NH)Gly2]Nociceptin(1-13)NH2
Catalog No.:BCC5701
CAS No.:213130-17-7
- RITA (NSC 652287)
Catalog No.:BCC2238
CAS No.:213261-59-7
- H-Pro-OMe.HCl
Catalog No.:BCC3022
CAS No.:2133-40-6
- 15,18-Dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1495
CAS No.:213329-45-4
- 18-Nor-4,15-dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1494
CAS No.:213329-46-5
Purvalanol induces endoplasmic reticulum stress-mediated apoptosis and autophagy in a time-dependent manner in HCT116 colon cancer cells.[Pubmed:25901510]
Oncol Rep. 2015 Jun;33(6):2761-70.
Purvalanol, a novel cyclin-dependent kinase inhibitor, is referred to as a strong apoptotic inducer which causes cell cycle arrest in various cancer cells such as prostate, breast and colon cancer cell lines. Various physiological and pathological conditions such as glucose starvation, inhibition of protein glycosylation and oxidative stress may cause an accumulation of unfolded proteins in the endoplasmic reticulum (ER), leading to the unfolded protein response (UPR) and autophagy. Lacking proteosomal function on aggregates of unfolded proteins, ER stress may induce autophagic machinery. Autophagy, an evolutionarily conserved process, is characterized by massive degradation of cytosolic contents. In the present study, our aim was to determine the time-dependent, ER-mediated apoptotic and autophagy induction of purvalanol in HCT 116 colon cancer cells. Fifteen micromoles of purvalanol induced a reduction in cell viability by 20 and 35% within 24 and 48 h, respectively. HCT 116 colon cancer cells were exposed to purvalanol, which activated ER stress via upregulation of PERK, IRE1alpha gene expression, eIF-2alpha phosphorylation and ATF-6 cleavage at early time-points in the HCT 116 colon cancer cells. Moreover, we determined that during purvalanol-mediated ER stress, autophagic machinery was also activated prior to apoptotic cell death finalization. Beclin-1 and Atg-5 expression levels were upregulated and LC3 was cleaved after a 6 h purvalanol treatment. Purvalanol induced mitochondrial membrane potential loss, caspase-7 and caspase-3 activation and PARP cleavage following a 48 h treatment. Thus, we conclude that the anticancer effect of purvalanol in HCT 116 cells was due to ER stress-mediated apoptosis; however, purvalanol triggered autophagy, which functions as a cell survival mechanism at early time-points.
Inhibition of autophagy by 3-MA potentiates purvalanol-induced apoptosis in Bax deficient HCT 116 colon cancer cells.[Pubmed:25088259]
Exp Cell Res. 2014 Oct 15;328(1):87-98.
The purine-derived analogs, roscovitine and Purvalanol Are selective synthetic inhibitors of cyclin-dependent kinases (CDKs) induced cell cycle arrest and lead to apoptotic cell death in various cancer cells. Although a number of studies investigated the molecular mechanism of each CDK inhibitor on apoptotic cell death mechanism with their therapeutic potential, their regulatory role on autophagy is not clarified yet. In this paper, our aim was to investigate molecular mechanism of CDK inhibitors on autophagy and apoptosis in wild type (wt) and Bax deficient HCT 116 cells. Exposure of HCT 116 wt and Bax(-/-) cells to roscovitine or purvalanol for 24h decreased cell viability in dose-dependent manner. However, Bax deficient HCT 116 cells were found more resistant against purvalanol treatment compared to wt cells. We also established that both CDK inhibitors induced apoptosis through activating mitochondria-mediated pathway in caspase-dependent manner regardless of Bax expression in HCT 116 colon cancer cells. Concomitantly, we determined that purvalanol was also effective on autophagy in HCT 116 colon cancer cells. Inhibition of autophagy by 3-MA treatment enhanced the purvalanol induced apoptotic cell death in HCT 116 Bax(-/-) cells. Our results revealed that mechanistic action of each CDK inhibitor on cell death mechanism differs. While purvalanol treatment activated apoptosis and autophagy in HCT 116 cells, roscovitine was only effective on caspase-dependent apoptotic pathway. Another important difference between two CDK inhibitors, although roscovitine treatment overcame Bax-mediated drug resistance in HCT 116 cells, purvalanol did not exert same effect.
Effects of cyclin-dependent kinase inhibitor Purvalanol B application on protein expression and developmental progression in intra-erythrocytic Plasmodium falciparum parasites.[Pubmed:25879664]
Malar J. 2015 Apr 8;14:147.
BACKGROUND: The 2013 Malaria World Report indicated that in 2012 there were approximately 207 million cases of malaria, which resulted in an estimated 627,000 malaria-related deaths. Due to the alarming resistance of these parasites to traditional anti-malarial treatments there is a pressing need to not only identify new anti-malarial compounds, but also to characterize the effect of compounds known to have an effect on the parasite life cycle. This study reports on effects of kinase inhibitor Purvalanol B administration on the growth and protein expression of Plasmodium falciparum late-stage trophozoites. METHODS: A SYBR(R) Green I parasite growth assay was used to measure the IC50 of Purvalanol B with P. falciparum (strain W2). Purvalanol B or DMSO control were applied to synchronized parasites 36 hours post invasion and parasites were incubated for 12 hours. Giemsa-stained blood smears were used to determine the effect of Purvalanol B on parasite growth, global quantitative proteomic analysis was used to examine differences in protein expression between Purvalanol B-treated and control parasites and results were confirmed by qPCR. RESULTS: There were no differences in parasitaemia between inhibitor-treated and control parasites. However, the ability of Purvalanol B-treated parasites to form schizonts was significantly reduced. Proteomic analysis detected 76 human proteins and 518 P. falciparum proteins (63 in control cultures only, 56 proteins in Purvalanol B cultures only, and 399 proteins in both cultures). Quantitative analysis of protein extracts revealed eight proteins that were up-regulated in the inhibitor-treated cultures, including several components of the parasite's proteasome complex and thioredoxin reductase. Two proteins appeared to be down-regulated, including a helicase and an RNA-binding protein. CONCLUSION: Purvalanol B application decreases the ability of late-stage P. falciparum trophozoites to form multinucleated schizonts and up-regulates proteasome subunits and proteins that contribute to redox homeostasis, which may indicate an increase in oxidative stress as a result of inhibitor application. While the efficacy of Purvalanol B is relatively low for use as an anti-malarial therapy, quantitative proteomic analysis may serve as a method of examining the action of drugs on the parasite and indicate the likelihood of future resistance development.
Placental passage of olomoucine II, but not purvalanol A, is affected by p-glycoprotein (ABCB1), breast cancer resistance protein (ABCG2) and multidrug resistance-associated proteins (ABCCs).[Pubmed:26364927]
Xenobiotica. 2016;46(5):416-23.
1. Purine cyclin-dependent kinase inhibitors have recently been recognised as promising candidates for the treatment of various cancers. While pharmacodynamic properties of these compounds are relatively well understood, their pharmacokinetics including possible interactions with placental transport systems have not been characterised to date. 2. In this study, we investigated transplacental passage of olomoucine II and Purvalanol A in rat focusing on possible role of p-glycoprotein (ABCB1), breast cancer resistance protein (ABCG2) and/or multidrug resistance-associated proteins (ABCCs). Employing the in situ method of dually perfused rat term placenta, we demonstrate transplacental passage of both olomoucine II and Purvalanol A against the concentration gradient in foetus-to-mother direction. Using several ATP-binding cassette (ABC) drug transporter inhibitors, we confirm the participation of ABCB1, ABCG2 and ABCCs transporters in the placental passage of olomoucine II, but not Purvalanol A. 3. Transplacental passage of olomoucine II and Purvalanol A from mother to foetus is significantly reduced by active transporters, restricting thereby foetal exposure and providing protection against harmful effects of these xenobiotics. Importantly, we demonstrate that in spite of their considerable structural similarity, the two molecules utilise distinct placental transport systems. These facts should be kept in mind when introducing these prospective anticancer candidates and/or their analogues into the clinical area.
The specificities of protein kinase inhibitors: an update.[Pubmed:12534346]
Biochem J. 2003 Apr 1;371(Pt 1):199-204.
We have previously examined the specificities of 28 commercially available compounds, reported to be relatively selective inhibitors of particular serine/threonine-specific protein kinases [Davies, Reddy, Caivano and Cohen (2000) Biochem. J. 351, 95-105]. In the present study, we have extended this analysis to a further 14 compounds. Of these, indirubin-3'-monoxime, SP 600125, KT 5823 and ML-9 were found to inhibit a number of protein kinases and conclusions drawn from their use in cell-based assays are likely to be erroneous. Kenpaullone, Alsterpaullone, Purvalanol, Roscovitine, pyrazolopyrimidine 1 (PP1), PP2 and ML-7 were more specific, but still inhibited two or more protein kinases with similar potency. Our results suggest that the combined use of Roscovitine and Kenpaullone may be useful for identifying substrates and physiological roles of cyclin-dependent protein kinases, whereas the combined use of Kenpaullone and LiCl may be useful for identifying substrates and physiological roles of glycogen synthase kinase 3. The combined use of SU 6656 and either PP1 or PP2 may be useful for identifying substrates of Src family members. Epigallocatechin 3-gallate, one of the main polyphenolic constituents of tea, inhibited two of the 28 protein kinases in the panel, dual-specificity, tyrosine-phosphorylated and regulated kinase 1A (DYRK1A; IC(50)=0.33 microM) and p38-regulated/activated kinase (PRAK; IC(50)=1.0 microM).
Cellular effects of purvalanol A: a specific inhibitor of cyclin-dependent kinase activities.[Pubmed:11857351]
Int J Cancer. 2002 Feb 20;97(6):761-9.
We have studied the effects of Purvalanol A on the cell cycle progression, proliferation and viability. In synchronized cells, Purvalanol A induced a reversible arrest the progression in G1 and G2 phase of the cell cycle, but did not prevent the completion of DNA synthesis in S-phase cells. The specificity of action of the drug was supported by the selective inhibition of the phosphorylation of cyclin-dependent kinase (cdk) substrates such as Rb and cyclin E. The cell contents of cyclins D1 and E were lower in cells incubated with Purvalanol A compared to controls, but the level of the cdk inhibitory protein p21(WAF1/CIP1) was increased, indicating that the drug did not cause a general inhibition of gene expression. Purvalanol A did not inhibit transcription under cell-free conditions. This compound, however, caused an inhibition of the estradiol-induced expression of an integrated luciferase gene, suggesting that cdk or related enzymes may participate in the regulation of the activity of certain promoters. When exponentially growing cells, both mouse fibroblasts and human cancer cell lines, were incubated with Purvalanol A for prolonged periods of time (24 hr), a lasting inhibition of cell proliferation as well as cell death were observed. In contrast, a 24 hr incubation of quiescent (non-transformed) cells with Purvalanol A did not prevent their resumption of cell cycle after removal of the drug.
ATP-site directed inhibitors of cyclin-dependent kinases.[Pubmed:10495356]
Curr Med Chem. 1999 Sep;6(9):859-75.
Cyclin-dependent kinases trigger and coordinate transitions between different phases the cell division cycle (CDK1, 2, 3, 4, 6, 7). They also play a role in apoptosis (CDK2), in neuronal cells (CDK5) and in the control of transcription (CDK 7, 8, 9). Intensive screening has lead to the recent identification of a series of chemical inhibitors of CDKs: olomoucine, roscovitine, purvalanol, CVT-313, flavopiridol, g-butyrolactone, indirubins, paullones and staurosporine. Some of these compounds display remarkable selectivities and efficiencies (IC50 < 25 nM). Many have been co-crystallised with CDK2 and their interactions with the kinase have been analysed in atomic detail. These inhibitors all act by competing with ATP for binding at the catalytic site. Most inhibitors present a flat heterocyclic ring system that occupies the purine binding pocket as well as form 2 or 3 hydrogen bonds with Glu-81 and Leu-83. The binding modes of these inhibitors are reviewed in this article. Knowledge of the CDK/inhibitor interactions will be of great help to design inhibitors with improved selectivity our potency as well as to generate affinity chromatography matrices for the purification and identification of their cellular targets. The potential use of CDK inhibitors is being extensively evaluated in cancer chemotherapy and other fields such as the cardiovascular domain (restenosis), dermatology (psoriasis), nephrology (glomerulonephritis) parasitology (unicellular parasites such as Plasmodium, Trypanosomes, Toxoplasm,.etc.), neurology (Alzheimer's disease) and viral infections (cytomegalovirus, H.I.V., herpes).
Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors.[Pubmed:9677190]
Science. 1998 Jul 24;281(5376):533-8.
Selective protein kinase inhibitors were developed on the basis of the unexpected binding mode of 2,6,9-trisubstituted purines to the adenosine triphosphate-binding site of the human cyclin-dependent kinase 2 (CDK2). By iterating chemical library synthesis and biological screening, potent inhibitors of the human CDK2-cyclin A kinase complex and of Saccharomyces cerevisiae Cdc28p were identified. The structural basis for the binding affinity and selectivity was determined by analysis of a three-dimensional crystal structure of a CDK2-inhibitor complex. The cellular effects of these compounds were characterized in mammalian cells and yeast. In the latter case the effects were characterized on a genome-wide scale by monitoring changes in messenger RNA levels in treated cells with high-density oligonucleotide probe arrays. Purine libraries could provide useful tools for analyzing a variety of signaling and regulatory pathways and may lead to the development of new therapeutics.


