Purvalanol BCDK1/CDK2/CDK4 inhibitor CAS# 212844-54-7 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- CDK inhibitor II
Catalog No.:BCC1464
CAS No.:1269815-17-9
- CGP60474
Catalog No.:BCC1474
CAS No.:164658-13-3
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 212844-54-7 | SDF | Download SDF |
| PubChem ID | 448991 | Appearance | Powder |
| Formula | C20H25ClN6O3 | M.Wt | 432.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NG 95 | ||
| Solubility | DMSO : ≥ 40 mg/mL (92.40 mM) *"≥" means soluble, but saturation unknown. | ||
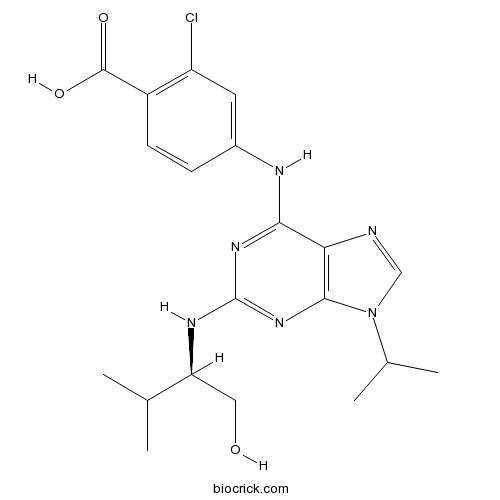
| Chemical Name | 2-chloro-4-[[2-[[(2R)-1-hydroxy-3-methylbutan-2-yl]amino]-9-propan-2-ylpurin-6-yl]amino]benzoic acid | ||
| SMILES | CC(C)C(CO)NC1=NC2=C(C(=N1)NC3=CC(=C(C=C3)C(=O)O)Cl)N=CN2C(C)C | ||
| Standard InChIKey | ZKDXRFMOHZVXSG-HNNXBMFYSA-N | ||
| Standard InChI | InChI=1S/C20H25ClN6O3/c1-10(2)15(8-28)24-20-25-17(16-18(26-20)27(9-22-16)11(3)4)23-12-5-6-13(19(29)30)14(21)7-12/h5-7,9-11,15,28H,8H2,1-4H3,(H,29,30)(H2,23,24,25,26)/t15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclin-dependent kinase inhibitor. IC50 values are 6, 6, 9, > 10,000, and 6 nM for cdc2/cyclin B, cdk2/cyclin A, cdk2/cyclin E, cdk4/cyclin D1 and cdk5-p35 respectively. Selective over a range of other protein kinases (IC50 > 10,000 nM). Shown to have antiproliferative properties, mediated by p42/p44 MAPK. |

Purvalanol B Dilution Calculator

Purvalanol B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.31 mL | 11.55 mL | 23.1 mL | 46.2 mL | 57.7501 mL |
| 5 mM | 0.462 mL | 2.31 mL | 4.62 mL | 9.24 mL | 11.55 mL |
| 10 mM | 0.231 mL | 1.155 mL | 2.31 mL | 4.62 mL | 5.775 mL |
| 50 mM | 0.0462 mL | 0.231 mL | 0.462 mL | 0.924 mL | 1.155 mL |
| 100 mM | 0.0231 mL | 0.1155 mL | 0.231 mL | 0.462 mL | 0.5775 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Purvalanol B is a selective inhibitor of CDK1, CDK2 and CDK4.
CDKs (cyclin-dependent kinases) family has the ability to regulate cell cycle.
Purvalanol B is reported as one of the most potent and selective CDK inhibitors. When tested with Chinese hamster lung fibroblast CCL39 cell line, treatment with Purvalanol B inhibited cells proliferate via targeting CKD1 which induced a G2/M block with a GI50 of 2.5 μM. In asynchronous cells, exposed to Purvalanol B led to an accumulation of cells in G2/M phase [1].
In a mouse model with NCI-H2228 subcutaneous xenograft, oral administration of ASP3026 caused significant reduction of phosphorylated ALK and tumor growth. 30 mg/kg/d ASP3026 for 2 weeks induced tumor regression by 78%. In mice injected with Karpas 299 cells, ASP3026 treatment caused remarkable lymphoma regression [2, 3].
Purvalanol B also interacts with p42/p44 MAPK proteins when tested with several mammalian cell lines including MCF-7 cell line [1].
References:
1.Kuromitsu S, Mori M, Shimada I, et al. Anti-tumor activity of ASP3026, a novel and selective ALK inhibitor of anaplastic lymphoma kinase (ALK). Annual Meeting of the American Association for Cancer Research (AACR), Orlando, FL. 2011.
2.Mori M, Ueno Y, Konagai S, et al. The Selective Anaplastic Lymphoma Receptor Tyrosine Kinase Inhibitor ASP3026 Induces Tumor Regression and Prolongs Survival in Non–Small Cell Lung Cancer Model Mice. Molecular cancer therapeutics, 2014, 13(2): 329-340.
3.George S K, Vishwamitra D, Manshouri R, et al. The ALK inhibitor ASP3026 eradicates NPM-ALK+ T-cell anaplastic large-cell lymphoma in vitro and in a systemic xenograft lymphoma model. Oncotarget, 2014, 5(14): 5750-5763.
- Purvalanol A
Catalog No.:BCC5654
CAS No.:212844-53-6
- Cowaxanthone B
Catalog No.:BCN3892
CAS No.:212842-64-3
- Kumokirine
Catalog No.:BCN2011
CAS No.:21284-20-8
- Kuramerine
Catalog No.:BCN1806
CAS No.:21284-19-5
- Ramage Linker,Fmoc-Suberol
Catalog No.:BCC2834
CAS No.:212783-75-0
- NG 52
Catalog No.:BCC1798
CAS No.:212779-48-1
- Xanthiazone
Catalog No.:BCN2544
CAS No.:212701-97-8
- PD184352 (CI-1040)
Catalog No.:BCC1112
CAS No.:212631-79-3
- PD 198306
Catalog No.:BCC7428
CAS No.:212631-61-3
- Nocistatin (human)
Catalog No.:BCC5732
CAS No.:212609-11-5
- Ebracteolatanolide A
Catalog No.:BCN3773
CAS No.:212563-72-9
- Oxaprozin
Catalog No.:BCC9109
CAS No.:21256-18-8
- (S)-(+)-Abscisic acid
Catalog No.:BCN2210
CAS No.:21293-29-8
- Tetrahymanol
Catalog No.:BCN6934
CAS No.:2130-17-8
- Tetrahymanol acetate
Catalog No.:BCN6933
CAS No.:2130-22-5
- Boc-Tyr(Bzl)-OH
Catalog No.:BCC3461
CAS No.:2130-96-3
- Org 37684
Catalog No.:BCC6291
CAS No.:213007-95-5
- Ceanothic acid
Catalog No.:BCN4918
CAS No.:21302-79-4
- [Phe1Ψ(CH2-NH)Gly2]Nociceptin(1-13)NH2
Catalog No.:BCC5701
CAS No.:213130-17-7
- RITA (NSC 652287)
Catalog No.:BCC2238
CAS No.:213261-59-7
- H-Pro-OMe.HCl
Catalog No.:BCC3022
CAS No.:2133-40-6
- 15,18-Dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1495
CAS No.:213329-45-4
- 18-Nor-4,15-dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1494
CAS No.:213329-46-5
- 1-Methyl-L-tryptophan
Catalog No.:BCN8341
CAS No.:21339-55-9
VMY-1-103, a dansylated analog of purvalanol B, induces caspase-3-dependent apoptosis in LNCaP prostate cancer cells.[Pubmed:20574155]
Cancer Biol Ther. 2010 Aug 15;10(4):320-5. Epub 2010 Aug 3.
The 2,6,9-trisubstituted purine group of cyclin dependent kinase inhibitors have the potential to be clinically relevant inhibitors of cancer cell proliferation. We have recently designed and synthesized a novel dansylated analog of Purvalanol B, termed VMY-1-103, that inhibited cell cycle progression in breast cancer cell lines more effectively than did Purvalanol B and allowed for uptake analyses by fluorescence microscopy. ErbB-2 plays an important role in the regulation of signal transduction cascades in a number of epithelial tumors, including prostate cancer (PCa). Our previous studies demonstrated that transgenic expression of activated ErbB-2 in the mouse prostate initiated PCa and either the overexpression of ErbB-2 or the addition of the ErbB-2/ErbB-3 ligand, heregulin (HRG), induced cell cycle progression in the androgen-responsive prostate cancer cell line, LNCaP. In the present study, we tested the efficacy of VMY-1-103 in inhibiting HRG-induced cell proliferation in LNCaP prostate cancer cells. At concentrations as low as 1 muM, VMY-1-103 increased both the proportion of cells in G(1) and p21(CIP1) protein levels. At higher concentrations (5 muM or 10 muM), VMY-1-103 induced apoptosis via decreased mitochondrial membrane polarity and induction of p53 phosphorylation, caspase-3 activity and PARP cleavage. Treatment with 10 muM Purvalanol B failed to either influence proliferation or induce apoptosis. Our results demonstrate that VMY-1-103 was more effective in inducing apoptosis in PCa cells than its parent compound, Purvalanol B, and support the testing of VMY-1-103 as a potential small molecule inhibitor of prostate cancer in vivo.
Effects of cyclin-dependent kinase inhibitor Purvalanol B application on protein expression and developmental progression in intra-erythrocytic Plasmodium falciparum parasites.[Pubmed:25879664]
Malar J. 2015 Apr 8;14:147.
BACKGROUND: The 2013 Malaria World Report indicated that in 2012 there were approximately 207 million cases of malaria, which resulted in an estimated 627,000 malaria-related deaths. Due to the alarming resistance of these parasites to traditional anti-malarial treatments there is a pressing need to not only identify new anti-malarial compounds, but also to characterize the effect of compounds known to have an effect on the parasite life cycle. This study reports on effects of kinase inhibitor Purvalanol B administration on the growth and protein expression of Plasmodium falciparum late-stage trophozoites. METHODS: A SYBR(R) Green I parasite growth assay was used to measure the IC50 of Purvalanol B with P. falciparum (strain W2). Purvalanol B or DMSO control were applied to synchronized parasites 36 hours post invasion and parasites were incubated for 12 hours. Giemsa-stained blood smears were used to determine the effect of Purvalanol B on parasite growth, global quantitative proteomic analysis was used to examine differences in protein expression between Purvalanol B-treated and control parasites and results were confirmed by qPCR. RESULTS: There were no differences in parasitaemia between inhibitor-treated and control parasites. However, the ability of Purvalanol B-treated parasites to form schizonts was significantly reduced. Proteomic analysis detected 76 human proteins and 518 P. falciparum proteins (63 in control cultures only, 56 proteins in Purvalanol B cultures only, and 399 proteins in both cultures). Quantitative analysis of protein extracts revealed eight proteins that were up-regulated in the inhibitor-treated cultures, including several components of the parasite's proteasome complex and thioredoxin reductase. Two proteins appeared to be down-regulated, including a helicase and an RNA-binding protein. CONCLUSION: Purvalanol B application decreases the ability of late-stage P. falciparum trophozoites to form multinucleated schizonts and up-regulates proteasome subunits and proteins that contribute to redox homeostasis, which may indicate an increase in oxidative stress as a result of inhibitor application. While the efficacy of Purvalanol B is relatively low for use as an anti-malarial therapy, quantitative proteomic analysis may serve as a method of examining the action of drugs on the parasite and indicate the likelihood of future resistance development.
p42/p44 MAPKs are intracellular targets of the CDK inhibitor purvalanol.[Pubmed:12226745]
Oncogene. 2002 Sep 19;21(42):6413-24.
Chemical inhibitors of cyclin-dependent kinases (CDKs) have a great therapeutic potential against various proliferative and neurodegenerative disorders. Intensive screening of a combinatorial chemistry library of 2,6,9-trisubstituted purines has led to the identification of purvalanol, one of the most potent and selective CDK inhibitors to date. In preliminary studies, this compound demonstrates definite anti-mitotic properties, consistent with its nanomolar range efficiency towards purified CDK1 and CDK2. However, the actual intracellular targets of purvalanol remain to be identified, and a method for the determination of its in vivo selectivity was developed. In this technique, cell extracts were screened for purvalanol-interacting proteins by affinity chromatography on immobilized inhibitor. In addition to CDK1, p42/p44 MAPK were found to be two major purvalanol-interacting proteins in five different mammalian cell lines (CCL39, PC12, HBL100, MCF-7 and Jurkat cells), suggesting the generality of the purvalanol/p42/p44 MAPK interaction. The Chinese hamster lung fibroblast cell line CCL39 was used as a model to investigate the anti-proliferative properties of purvalanol. The compound inhibited cell growth with a GI(50) value of 2.5 microM and induced a G2/M block when added to exponentially growing cells. It did not appear to trigger massive activation of caspase. We next tested whether CDKs and p42/p44 MAPK were actually targeted by the compound in vivo. p42/p44 MAPK activity was visualized using an Elk-Gal4 luciferase reporter system and CDK1 activity was detected by the phosphonucleolin level. When cells were treated with purvalanol, p42/p44 MAPK and CDK1 activities were inhibited in a dose-dependent manner. Furthermore, purvalanol inhibited the nuclear accumulation of p42/p44 MAPK, an event dependent on the catalytic activity of these kinases. We conclude that the anti-proliferative properties of purvalanol are mediated by inhibition of both p42/p44 MAPK and CDKs. These observations highlight the potency of moderate selectivity compounds and encourage the search for new therapeutics which simultaneously target distinct but relevant pathways of cell proliferation.
ATP-site directed inhibitors of cyclin-dependent kinases.[Pubmed:10495356]
Curr Med Chem. 1999 Sep;6(9):859-75.
Cyclin-dependent kinases trigger and coordinate transitions between different phases the cell division cycle (CDK1, 2, 3, 4, 6, 7). They also play a role in apoptosis (CDK2), in neuronal cells (CDK5) and in the control of transcription (CDK 7, 8, 9). Intensive screening has lead to the recent identification of a series of chemical inhibitors of CDKs: olomoucine, roscovitine, purvalanol, CVT-313, flavopiridol, g-butyrolactone, indirubins, paullones and staurosporine. Some of these compounds display remarkable selectivities and efficiencies (IC50 < 25 nM). Many have been co-crystallised with CDK2 and their interactions with the kinase have been analysed in atomic detail. These inhibitors all act by competing with ATP for binding at the catalytic site. Most inhibitors present a flat heterocyclic ring system that occupies the purine binding pocket as well as form 2 or 3 hydrogen bonds with Glu-81 and Leu-83. The binding modes of these inhibitors are reviewed in this article. Knowledge of the CDK/inhibitor interactions will be of great help to design inhibitors with improved selectivity our potency as well as to generate affinity chromatography matrices for the purification and identification of their cellular targets. The potential use of CDK inhibitors is being extensively evaluated in cancer chemotherapy and other fields such as the cardiovascular domain (restenosis), dermatology (psoriasis), nephrology (glomerulonephritis) parasitology (unicellular parasites such as Plasmodium, Trypanosomes, Toxoplasm,.etc.), neurology (Alzheimer's disease) and viral infections (cytomegalovirus, H.I.V., herpes).
Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors.[Pubmed:9677190]
Science. 1998 Jul 24;281(5376):533-8.
Selective protein kinase inhibitors were developed on the basis of the unexpected binding mode of 2,6,9-trisubstituted purines to the adenosine triphosphate-binding site of the human cyclin-dependent kinase 2 (CDK2). By iterating chemical library synthesis and biological screening, potent inhibitors of the human CDK2-cyclin A kinase complex and of Saccharomyces cerevisiae Cdc28p were identified. The structural basis for the binding affinity and selectivity was determined by analysis of a three-dimensional crystal structure of a CDK2-inhibitor complex. The cellular effects of these compounds were characterized in mammalian cells and yeast. In the latter case the effects were characterized on a genome-wide scale by monitoring changes in messenger RNA levels in treated cells with high-density oligonucleotide probe arrays. Purine libraries could provide useful tools for analyzing a variety of signaling and regulatory pathways and may lead to the development of new therapeutics.


