CDK inhibitor IICAS# 1269815-17-9 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1269815-17-9 | SDF | Download SDF |
| PubChem ID | 50991505 | Appearance | Powder |
| Formula | C18H19ClFN3O2 | M.Wt | 363.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CDK inhibitor II | ||
| Solubility | DMSO : ≥ 100 mg/mL (274.87 mM) *"≥" means soluble, but saturation unknown. | ||
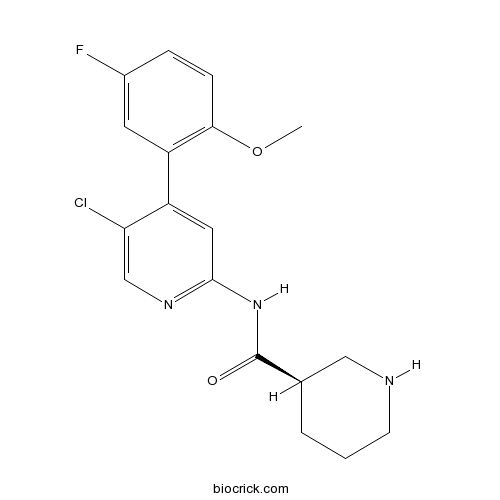
| Chemical Name | (3R)-N-[5-chloro-4-(5-fluoro-2-methoxyphenyl)pyridin-2-yl]piperidine-3-carboxamide | ||
| SMILES | COC1=C(C=C(C=C1)F)C2=CC(=NC=C2Cl)NC(=O)C3CCCNC3 | ||
| Standard InChIKey | AHMKHNVZGOQLRQ-LLVKDONJSA-N | ||
| Standard InChI | InChI=1S/C18H19ClFN3O2/c1-25-16-5-4-12(20)7-14(16)13-8-17(22-10-15(13)19)23-18(24)11-3-2-6-21-9-11/h4-5,7-8,10-11,21H,2-3,6,9H2,1H3,(H,22,23,24)/t11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CDK-IN-2 is a potent and specific CDK9 inhibitor with IC50 of <8 nM, extracted from reference 1, example 4.
IC50 Value: <8 nM [1]
Target: CDK9
In vitro:
In vivo: References: | |||||

CDK inhibitor II Dilution Calculator

CDK inhibitor II Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7486 mL | 13.7431 mL | 27.4861 mL | 54.9722 mL | 68.7153 mL |
| 5 mM | 0.5497 mL | 2.7486 mL | 5.4972 mL | 10.9944 mL | 13.7431 mL |
| 10 mM | 0.2749 mL | 1.3743 mL | 2.7486 mL | 5.4972 mL | 6.8715 mL |
| 50 mM | 0.055 mL | 0.2749 mL | 0.5497 mL | 1.0994 mL | 1.3743 mL |
| 100 mM | 0.0275 mL | 0.1374 mL | 0.2749 mL | 0.5497 mL | 0.6872 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CDK inhibitor II is a potent CDK inhibitor.
- Tetrahydro tanshinone I
Catalog No.:BCN2602
CAS No.:126979-84-8
- Methylenedihydrotanshinquinone
Catalog No.:BCN3221
CAS No.:126979-81-5
- Sar-[D-Phe8]-des-Arg9-Bradykinin
Catalog No.:BCC5996
CAS No.:126959-88-4
- LGX818
Catalog No.:BCC4184
CAS No.:1269440-17-6
- MK 1903
Catalog No.:BCC6242
CAS No.:1268882-43-4
- 3-Methoxy-5-heneicosylphenol
Catalog No.:BCN6147
CAS No.:126882-76-6
- Ssioriside
Catalog No.:BCN6146
CAS No.:126882-53-9
- MC 1046
Catalog No.:BCC1733
CAS No.:126860-83-1
- (-)-JQ1
Catalog No.:BCC3603
CAS No.:1268524-71-5
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- 8alpha-Acetoxyarglabin
Catalog No.:BCN7315
CAS No.:126829-70-7
- 13-Acetoxy-3beta-hydroxygermacra-1(10)E,4E,7(11)-trien-12,6alpha-olide
Catalog No.:BCN7314
CAS No.:126829-66-1
- 5-Hydroxy-1,7-bis(4-hydroxyphenyl)heptan-3-yl acetate
Catalog No.:BCN6586
CAS No.:1269839-24-8
- 1-(3,4-Dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane-3,5-diyl diacetate
Catalog No.:BCN6572
CAS No.:1269839-26-0
- Locustatachykinin I
Catalog No.:BCC5926
CAS No.:126985-97-5
- Hydroxyurea
Catalog No.:BCC4912
CAS No.:127-07-1
- Sodium acetate
Catalog No.:BCC7587
CAS No.:127-09-3
- Taraxerol
Catalog No.:BCN6148
CAS No.:127-22-0
- Pimaric acid
Catalog No.:BCN6149
CAS No.:127-27-5
- Lasiocarpine N-oxide
Catalog No.:BCN2002
CAS No.:127-30-0
- Lutein
Catalog No.:BCN6151
CAS No.:127-40-2
- Vitamin A Acetate
Catalog No.:BCC4748
CAS No.:127-47-9
- 2,2-Bis(4-hydroxy-3-isopropylphenyl)propane
Catalog No.:BCC8494
CAS No.:127-54-8
- Sulfacetamide Sodium
Catalog No.:BCC4383
CAS No.:127-56-0
Cellular response to antitumor cis-Dichlorido platinum(II) complexes of CDK inhibitor Bohemine and its analogues.[Pubmed:22250642]
Chem Res Toxicol. 2012 Feb 20;25(2):500-9.
The cellular and molecular pharmacology of the new class of anticancer drugs, in which the CDK inhibitor bohemine and its analogues are coordinated to Pt(II) to form cisplatin derivatives, was investigated. The results revealed the unique anticancer profile of a cisplatin-derived platinum(II) dichlorido complex involving N(7)-coordinated bohemine (C1). Although the IC(50) values were approximately 6-fold higher for C1 than for cisplatin in cisplatin-sensitive tumor cells, the tumor cells in which C1 was also active are those which acquired resistance to cisplatin. In addition, among the novel conjugates of bohemine and its analogues with cisplatin, marked selectivity of C1 for tumor cells relative to the nontumorigenic, normal cells was observed. However, coordination of bohemine to platinum in C1 considerably reduced one of the dual functionalities anticipated to be effective after C1 reaches the nucleus. Further studies performed in the cells with wt p53 status show differences between cisplatin and C1 at the level of cell cycle regulation. Impedance-based real-time monitoring of the effects of C1 and cisplatin on cell growth supported the thesis that critical differences exist in the rate and mechanisms of cell kill caused by the two agents and that C1 was a more potent inducer of apoptosis and/or necrosis than cisplatin. The results also showed that the distinct differences in cell killing observed for C1 and cisplatin might be associated with processes at the DNA level. The DNA binding experiments carried out in a cell-free medium demonstrated that modification reactions resulting in the irreversible coordination of C1 to DNA were slower than that of cisplatin. Transcription mapping experiments and determination of interstrand cross-linking efficiency of C1 suggested that several aspects of DNA binding mode of C1 and cisplatin were similar. It was concluded that C1 remains a promising prototype of compounds for the generation of novel drug candidates with cytotoxicity profiles different from those of the platinum drugs currently in use.
CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment.[Pubmed:25501126]
Clin Cancer Res. 2015 Mar 1;21(5):995-1001.
PURPOSE: The G1-S checkpoint of the cell cycle is frequently dysregulated in breast cancer. Palbociclib (PD0332991) is an oral inhibitor of CDK4/6. Based upon preclinical/phase I activity, we performed a phase II, single-arm trial of palbociclib in advanced breast cancer. EXPERIMENTAL DESIGN: Eligible patients had histologically confirmed, metastatic breast cancer positive for retinoblastoma (Rb) protein and measureable disease. Palbociclib was given at 125 mg orally on days 1 to 21 of a 28-day cycle. Primary objectives were tumor response and tolerability. Secondary objectives included progression-free survival (PFS) and assessment of Rb expression/localization, KI-67, p16 loss, and CCND1 amplification. RESULTS: Thirty-seven patients were enrolled; 84% hormone-receptor (HR)(+)/Her2(-), 5% HR(+)/Her2(+), and 11% HR(-)/Her2(-), with a median of 2 prior cytotoxic regimens. Two patients had partial response (PR) and 5 had stable disease >/= 6 months for a clinical benefit rate (CBR = PR + 6moSD) of 19% overall, 21% in HR(+), and 29% in HR(+)/Her2(-) who had progressed through >/=2 prior lines of hormonal therapy. Median PFS overall was 3.7 months [95% confidence interval (CI), 1.9-5.1], but significantly longer for those with HR(+) versus HR(-) disease (P = 0.03) and those who had previously progressed through endocrine therapy for advanced disease (P = 0.02). Grade 3/4 toxicities included neutropenia (51%), anemia (5%), and thrombocytopenia (22%). Twenty-four percent had treatment interruption and 51% had dose reduction, all for cytopenias. No biomarker identified a sensitive tumor population. CONCLUSIONS: Single-agent palbociclib is well tolerated and active in patients with endocrine-resistant, HR(+), Rb-positive breast cancer. Cytopenias were uncomplicated and easily managed with dose reduction.
The CDK inhibitor AT7519M in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) and mantle cell lymphoma. A Phase II study of the Canadian Cancer Trials Group.[Pubmed:27750483]
Leuk Lymphoma. 2017 Jun;58(6):1358-1365.
AT7519M is a small molecule inhibitor of cyclin-dependent kinases 1, 2, 4, 5, and 9 with in vitro activity against lymphoid malignancies. In two concurrent Phase II trials, we evaluated AT7519M in relapsed or refractory chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL) using the recommended Phase II dosing of 27 mg/m(2) twice weekly for 2 of every 3 weeks. Primary objective was objective response rate (ORR). Nineteen patients were accrued (7 CLL, 12 MCL). Four CLL patients achieved stable disease (SD). Two MCL patients achieved partial response (PR), and 6 had SD. One additional MCL patient with SD subsequently achieved PR 9 months after completion of AT7519M. Tumor lysis syndrome was not reported. In conclusion, AT7519M was safely administered to patients with relapsed/refractory CLL and MCL. In CLL, some patients had tumor reductions, but the ORR was low. In MCL, activity was noted with ORR of 27%.
Conformation and recognition of DNA damaged by antitumor cis-dichlorido platinum(II) complex of CDK inhibitor bohemine.[Pubmed:24675180]
Eur J Med Chem. 2014 May 6;78:54-64.
A substitution of the ammine ligands of cisplatin, cis-[Pt(NH3)2Cl2], for cyclin dependent kinase (CDK) inhibitor bohemine (boh), [2-(3-hydroxypropylamino)-6-benzylamino-9-isopropylpurine], results in a compound, cis-[Pt(boh)2Cl2] (C1), with the unique anticancer profile which may be associated with some features of the damaged DNA and/or its cellular processing (Travnicek Z et al. (2003) J Inorg Biochem94, 307-316; Liskova B (2012) Chem Res Toxicol25, 500-509). A combination of biochemical and molecular biology techniques was used to establish mechanistic differences between cisplatin and C1 with respect to the DNA damage they produce and their interactions with critical DNA-binding proteins, DNA-processing enzymes and glutathione. The results show that replacement of the NH3 groups in cisplatin by bohemine modulates some aspects of the mechanism of action of C1. More specifically, the results of the present work are consistent with the thesis that, in comparison with cisplatin, effects of other factors, such as: (i) slower rate of initial binding of C1 to DNA; (ii) the lower efficiency of C1 to form bifunctional adducts; (iii) the reduced bend of longitudinal DNA axis induced by the major 1,2-GG intrastrand cross-link of C1; (iv) the reduced affinity of HMG domain proteins to the major adduct of C1; (v) the enhanced efficiency of the DNA adducts of C1 to block DNA polymerization and to inhibit transcription activity of human RNA pol II and RNA transcription; (vi) slower rate of the reaction of C1 with glutathione, may partially contribute to the unique activity of C1.


