Pimaric acidCAS# 127-27-5 |
- Continentalic acid
Catalog No.:BCN6526
CAS No.:19889-23-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 127-27-5 | SDF | Download SDF |
| PubChem ID | 220338 | Appearance | Powder |
| Formula | C20H30O2 | M.Wt | 302.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
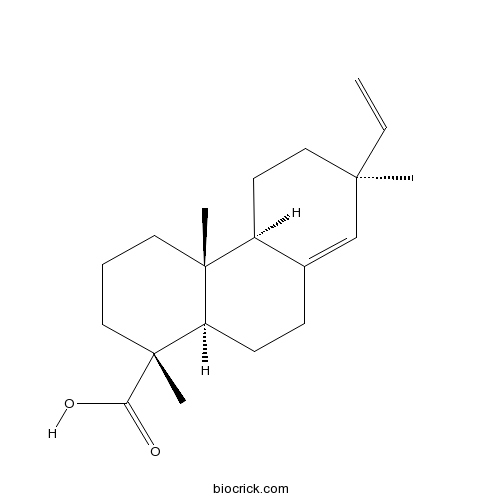
| Chemical Name | (1R,4aR,4bS,7S,10aR)-7-ethenyl-1,4a,7-trimethyl-3,4,4b,5,6,9,10,10a-octahydro-2H-phenanthrene-1-carboxylic acid | ||
| SMILES | CC1(CCC2C(=C1)CCC3C2(CCCC3(C)C(=O)O)C)C=C | ||
| Standard InChIKey | MHVJRKBZMUDEEV-APQLOABGSA-N | ||
| Standard InChI | InChI=1S/C20H30O2/c1-5-18(2)12-9-15-14(13-18)7-8-16-19(15,3)10-6-11-20(16,4)17(21)22/h5,13,15-16H,1,6-12H2,2-4H3,(H,21,22)/t15-,16+,18+,19+,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pimaric acid has potent anti-atherosclerotic activity with inhibitory action on matrix metalloproteinase-9 production and cell migration in TNF-α-induced human aortic smooth muscle cells. 2. 4-epi-Pimaric acid shows antibacterial, anti-biofilm and anti-inflammatory potency , can be exploited for therapeutic application in oral microbial infections. |
| Targets | NF-kB | p38MAPK | MMP(e.g.TIMP) | TNF-α | Calcium Channel | Potassium Channel | p65 |

Pimaric acid Dilution Calculator

Pimaric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3058 mL | 16.5289 mL | 33.0579 mL | 66.1157 mL | 82.6446 mL |
| 5 mM | 0.6612 mL | 3.3058 mL | 6.6116 mL | 13.2231 mL | 16.5289 mL |
| 10 mM | 0.3306 mL | 1.6529 mL | 3.3058 mL | 6.6116 mL | 8.2645 mL |
| 50 mM | 0.0661 mL | 0.3306 mL | 0.6612 mL | 1.3223 mL | 1.6529 mL |
| 100 mM | 0.0331 mL | 0.1653 mL | 0.3306 mL | 0.6612 mL | 0.8264 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Taraxerol
Catalog No.:BCN6148
CAS No.:127-22-0
- Sodium acetate
Catalog No.:BCC7587
CAS No.:127-09-3
- Hydroxyurea
Catalog No.:BCC4912
CAS No.:127-07-1
- Locustatachykinin I
Catalog No.:BCC5926
CAS No.:126985-97-5
- 1-(3,4-Dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane-3,5-diyl diacetate
Catalog No.:BCN6572
CAS No.:1269839-26-0
- 5-Hydroxy-1,7-bis(4-hydroxyphenyl)heptan-3-yl acetate
Catalog No.:BCN6586
CAS No.:1269839-24-8
- CDK inhibitor II
Catalog No.:BCC1464
CAS No.:1269815-17-9
- Tetrahydro tanshinone I
Catalog No.:BCN2602
CAS No.:126979-84-8
- Methylenedihydrotanshinquinone
Catalog No.:BCN3221
CAS No.:126979-81-5
- Sar-[D-Phe8]-des-Arg9-Bradykinin
Catalog No.:BCC5996
CAS No.:126959-88-4
- LGX818
Catalog No.:BCC4184
CAS No.:1269440-17-6
- MK 1903
Catalog No.:BCC6242
CAS No.:1268882-43-4
- Lasiocarpine N-oxide
Catalog No.:BCN2002
CAS No.:127-30-0
- Lutein
Catalog No.:BCN6151
CAS No.:127-40-2
- Vitamin A Acetate
Catalog No.:BCC4748
CAS No.:127-47-9
- 2,2-Bis(4-hydroxy-3-isopropylphenyl)propane
Catalog No.:BCC8494
CAS No.:127-54-8
- Sulfacetamide Sodium
Catalog No.:BCC4383
CAS No.:127-56-0
- Sulfamerazine sodium salt
Catalog No.:BCC5205
CAS No.:127-58-2
- Sulfisoxazole
Catalog No.:BCC4860
CAS No.:127-69-5
- Sulfamerazine
Catalog No.:BCC4854
CAS No.:127-79-7
- Beta-Pinene
Catalog No.:BCC8302
CAS No.:127-91-3
- 4,9-Dimethoxycanthin-6-one
Catalog No.:BCN3107
CAS No.:1270001-72-3
- CGP 42112
Catalog No.:BCC5921
CAS No.:127060-75-7
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
Retinoic acid receptor agonist activity of naturally occurring diterpenes.[Pubmed:24799257]
Bioorg Med Chem. 2014 Jun 15;22(12):3204-12.
Recent accumulating evidence indicates that all-trans retinoic acid (ATRA) may be useful for preventing or treating inflammation, allergy, and autoimmune diseases, despite its severe side effects. In this study, screening of 99 crude drugs for retinoic acid receptor (RAR) ligands by luciferase reporter assay demonstrated that the methanol extract of Aralia cordata Rhizoma most effectively activates the transcriptional activity of RARalpha. Pimaradienoic acid (ent-pimara-8(14),15-dien-19-oic acid) was subsequently isolated as the constituent capable of activating RAR. Pimaric acid and abietic acid, which have similar structures to pimaradienoic acid, were also found to be novel RAR agonists, although abietic acid only slightly activated peroxisome proliferator-activated receptor gamma. These three natural RAR agonists with diterpene structures, while structurally different from ATRA, were able to increase the mRNA levels of the constitutive androstane receptor in HepG2 cells, induce F9 cell differentiation followed by Cyp26a1 mRNA expression, and differentiate HL-60 cells via RAR activation in a different manner from ATRA. These results demonstrate that some diterpenes exist as naturally occurring RAR agonists and that the differences in chemical structure between ATRA and these diterpenes may induce distinct gene activation and a specific cellular response.
Effects of ketamine and its metabolites on ion currents in differentiated hippocampal H19-7 neuronal cells and in HEK293T cells transfected with alpha-hslo subunit.[Pubmed:23227486]
Neurotoxicology. 2012 Oct;33(5):1058-66.
Ketamine (KT), a dissociative anesthetic, is known to induce schizophrenia-like psychosis. The percentage of KT abuse has recently grown fast despite KT being a controlled drug. The mechanism of KT actions is related to the inhibition of NMDA receptors. Whether KT produces other effects on ion currents in hippocampal neurons remains unclear. In this study, we attempted to evaluate the possible effects of KT and other related compounds on ion currents in hippocampal neuron-derived H19-7 cells. This drug exerted an inhibitory effect on Ca(2+)-activated K(+) current (IK(Ca)) in these cells with an IC(50) value of 274 muM. Pimaric acid (30 muM) or abietic acid (30 muM), known to stimulate large-conductance Ca(2+)-activated K(+) channels, reversed KT-induced inhibition of I(K)(Ca). In HEK293T cells expressing a-humans low poke, KT-induced inhibition of I(K)(Ca) still existed. Dehydronorketamine (300 muM) had little or no effect on the IK(Ca) amplitude, while norketamine (300 muM) slightly but significantly suppressed it. In inside-out configuration, KT applied to the intracellular face of the membrane did not alter single channel conductance of large-conductance Ca(2+)-activated K(+) (BKCa) channels; however, it did significantly reduce the probability of channel openings. Addition of KT was effective in depressing the peak amplitude of voltage-gated Na(+) current. Moreover, the presence of KT was noted to enhance the amplitude of membrane electroporation-induced inward currents (IMEP) in differentiated H19-7 cells. KT-stimulated IMEP was reversed by further application of LaCl(3) (100 muM), but not by NMDA (30 muM). The modulations by this compound of ion channels may contribute to the underlying mechanisms through which KT and its metabolites influence the electrical behavior of hippocampal neurons if similar findings occur in vivo.
4-epi-Pimaric acid: a phytomolecule as a potent antibacterial and anti-biofilm agent for oral cavity pathogens.[Pubmed:21594714]
Eur J Clin Microbiol Infect Dis. 2012 Feb;31(2):149-59.
The present study focused on the antibacterial and biofilm inhibitory potential of 4-epi-Pimaric acid isolated from aerial parts (stem and leaves) of Aralia cachemirica L. (Araliaceae) against oral cavity pathogens. 4-epi-Pimaric acid exhibited minimum inhibitory concentration (MIC) in the range of 4-16 mug/ml and minimum bactericidal concentration (MBC) two- to four-folds higher than MIC. There was significant inhibition in the biofilm formation by Streptococcus mutans on the saliva coated surface (P < 0.05), and confocal microscopy revealed that 4-epi-Pimaric acid inhibited the clumping and attachment of S. mutans. At 8 x MIC concentration, it significantly prevented the pH drop and reduced S. mutans biofilms (P < 0.05). Increased propidium iodide staining and leakage of 260- and 280-nm absorbing material by 4-epi-Pimaric acid treated cells of S. mutans suggested that it probably causes disruption of the cytoplasmic membrane structure. It also exhibited significant suppression of TNF-alpha expression in human neutrophils, suggestive of its anti-inflammatory activity. Furthermore, the compound was found to be significantly safe (IC(50) >100 mug/ml) in the MTT assay on AML-12 cell lines. In conclusion, 4-epi-Pimaric acid showed promising antibacterial, anti-biofilm and anti-inflammatory potency and this compound can be exploited for therapeutic application in oral microbial infections.
Pimaric acid from Aralia cordata has an inhibitory effect on TNF-alpha-induced MMP-9 production and HASMC migration via down-regulated NF-kappaB and AP-1.[Pubmed:22705379]
Chem Biol Interact. 2012 Aug 30;199(2):112-9.
Many studies have indicated that activation of matrix metalloproteinase (MMP)-9 and smooth muscle cell (SMC) migration are involved in neointimal formation and atherosclerosis. In this study, we revealed that Pimaric acid (PiMA) purified from Aralia cordata had an inhibitory effect on MMP-9 production and migration of human aortic smooth muscle cells (HASMCs) induced by tumor necrosis factor (TNF)-alpha. Down-regulated MMP-9 mRNA transcription was detected in PiMA-treated cells using RT-PCR and the luciferase-tagged MMP-9 promoter assay. Results of an electrophoretic mobility shift assay indicated that PiMA-treated HASMCs showed decreased binding activity of nuclear factor (NF)-kappaB and activator protein-1 transcription factors. A Western-blot analysis using nuclear extract demonstrated that PiMA reduced the levels of NF-kappaB p65, c-Fos, p-c-Jun, Jun-D, and p-ATF2 proteins in the nucleus. In addition, TNF-alpha stimulated mitogen activated protein kinase (MAPK) containing extracellular signal regulated kinase 1 and 2, p38, and c-Jun N-terminal kinase was inhibited by PiMA. Using the Transwell system, we found that PiMA inhibited TNF-alpha stimulated HASMC migration/invasion in a dose-dependent manner. To confirm whether MAPK mediated MMP-9 expression, we used MAPK inhibitors including U0126, SB253580, and SP600125 and found that those inhibitors reduced MMP-9 expression and HASMC migration/invasion. These results suggest that PiMA has potent anti-atherosclerotic activity with inhibitory action on MMP-9 production and cell migration in TNF-alpha-induced HASMCs.


