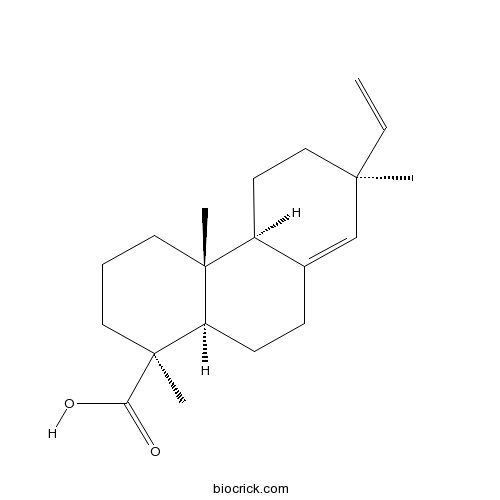
Continentalic acidCAS# 19889-23-7 |
- Pimaric acid
Catalog No.:BCN6149
CAS No.:127-27-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19889-23-7 | SDF | Download SDF |
| PubChem ID | 23616873 | Appearance | White powder |
| Formula | C20H30O2 | M.Wt | 302.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Pimaradienoic acid | ||
| Solubility | Soluble in methan | ||
| Chemical Name | (1S,4aR,4bS,7S,10aR)-7-ethenyl-1,4a,7-trimethyl-3,4,4b,5,6,9,10,10a-octahydro-2H-phenanthrene-1-carboxylic acid | ||
| SMILES | CC1(CCC2C(=C1)CCC3C2(CCCC3(C)C(=O)O)C)C=C | ||
| Standard InChIKey | MHVJRKBZMUDEEV-MSJBJCGKSA-N | ||
| Standard InChI | InChI=1S/C20H30O2/c1-5-18(2)12-9-15-14(13-18)7-8-16-19(15,3)10-6-11-20(16,4)17(21)22/h5,13,15-16H,1,6-12H2,2-4H3,(H,21,22)/t15-,16+,18+,19+,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Continentalic acid and kaurenoic acid are quality control markers in Aralia continentalis. 2. Continentalic acid shows moderate cytotoxicity against A-549 (lung), THP-1 (leukemia) and MCF-7 (breast) cell lines. 3. Continentalic acid exerts significant anti-inflammatory activity. 4. Continentalic acid can efficiently induces apoptosis and is a good candidate for further evaluation as an effective chemotherapeutic agent. 5. Continentalic acid has minimum inhibitory concentrations (MICs) of approximately 8-16 microg/mL against S. aureus, including the MSSA and MRSA standard strains. |
| Targets | COX | PGE | NOS | NO | PARP | Bcl-2/Bax | Caspase | Antifection |

Continentalic acid Dilution Calculator

Continentalic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3058 mL | 16.5289 mL | 33.0579 mL | 66.1157 mL | 82.6446 mL |
| 5 mM | 0.6612 mL | 3.3058 mL | 6.6116 mL | 13.2231 mL | 16.5289 mL |
| 10 mM | 0.3306 mL | 1.6529 mL | 3.3058 mL | 6.6116 mL | 8.2645 mL |
| 50 mM | 0.0661 mL | 0.3306 mL | 0.6612 mL | 1.3223 mL | 1.6529 mL |
| 100 mM | 0.0331 mL | 0.1653 mL | 0.3306 mL | 0.6612 mL | 0.8264 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Humulene epoxide II
Catalog No.:BCN4873
CAS No.:19888-34-7
- Alisol A
Catalog No.:BCN3455
CAS No.:19885-10-0
- H-Phe(2-F)-OH
Catalog No.:BCC3222
CAS No.:19883-78-4
- H-D-Phg-OMe.HCl
Catalog No.:BCC3314
CAS No.:19883-41-1
- Z-D-Tyr(tBu)-OH.DCHA
Catalog No.:BCC2744
CAS No.:198828-72-7
- (7R)-Methoxy-8-epi-matairesinol
Catalog No.:BCN7582
CAS No.:198827-23-5
- Merimepodib
Catalog No.:BCC4128
CAS No.:198821-22-6
- Windorphen
Catalog No.:BCC6486
CAS No.:19881-70-0
- Bavachin
Catalog No.:BCN4872
CAS No.:19879-32-4
- Bavachinin
Catalog No.:BCN4871
CAS No.:19879-30-2
- 1,3-Dicaffeoylquinic acid
Catalog No.:BCN2972
CAS No.:19870-46-3
- MLCK inhibitor peptide
Catalog No.:BCC5852
CAS No.:198694-74-5
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Nagilactone B
Catalog No.:BCN4049
CAS No.:19891-51-1
- Jaborosalactone D
Catalog No.:BCN7946
CAS No.:19891-82-8
- 29-Nor-20-oxolupeol
Catalog No.:BCN6678
CAS No.:19891-85-1
- Kaempferol 3-O-beta-sophoroside
Catalog No.:BCN3336
CAS No.:19895-95-5
- Syringaresinol diacetate
Catalog No.:BCN4874
CAS No.:1990-77-8
- Dihydromethysticin
Catalog No.:BCN2476
CAS No.:19902-91-1
- Bakkenolide A
Catalog No.:BCN5402
CAS No.:19906-72-0
- Maackiain
Catalog No.:BCN1236
CAS No.:19908-48-6
- H-Phe(4-Me)-OH
Catalog No.:BCC3270
CAS No.:1991-87-3
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- T 98475
Catalog No.:BCC7395
CAS No.:199119-18-1
Continentalic acid from Aralia continentalis shows activity against methicillin-resistant Staphylococcus aureus.[Pubmed:16619343]
Phytother Res. 2006 Jun;20(6):511-4.
In a continuing search for compounds with antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA), a chloroform extract of roots of Aralia continentalis was found to contain Continentalic acid (CA, C(20)H(30)O(2)), a diterpenic acid. This compound exhibited potent activity against standard methicillin-susceptible Staphylococcus aureus (MSSA) as well as clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). It was determined that Continentalic acid had minimum inhibitory concentrations (MICs) of approximately 8-16 microg/mL against S. aureus, including the MSSA and MRSA standard strains. Therefore, the results obtained in this study suggest that Continentalic acid might have potential as an adjunct in the treatment of antibiotic-resistant bacteria.
Ultra-performance convergence chromatography for the quantitative determination of bioactive compounds in Aralia continentalis Kitagawa as quality control markers.[Pubmed:28306202]
J Sep Sci. 2017 May;40(9):2071-2079.
A rapid ultra-performance convergence chromatography method was developed for the quantitative determination of bioactive compounds in Aralia continentalis as quality control markers. Quantitative analysis indicated the presence of two major bioactive compounds: diterpenoid acids Continentalic acid and kaurenoic acid. Using a Torus 1-aminoanthracene column, Continentalic acid and kaurenoic acid were separated in less than 8 min. The method was validated with respect to precision, accuracy, and linearity according to the International Conference on Harmonization guidelines. The optimized method exhibited a good linear correlation (r(2) > 0.996), excellent precision (RSD < 1.0%), and acceptable recoveries (99.97-100.26%). Limits of detection for Continentalic acid and kaurenoic acid were 0.068 and 0.097 mug/mL, respectively, while their corresponding limits of quantitation were 0.207 and 0.295 mug/mL. The system performance of ultra-performance convergence chromatography was compared with that of conventional high-performance liquid chromatography with respect to analysis time and efficiency. The proposed method was found to be reliable and convenient for the quantitative analysis of Continentalic acid and kaurenoic acid in A. continentalis from South Korea and A. pubescens from China. This study is expected to serve as a guideline for the quality control of Aralia continentalis.
In vitro Cytotoxicity of Methanol Extract from Aerial Parts of Aralia cachemirica and Purified Continentalic Acid.[Pubmed:26997711]
Indian J Pharm Sci. 2015 Nov-Dec;77(6):792-5.
The present study was designed to evaluate the in vitro cytotoxic effect of methanol extract of aerial parts including stems, leaves and twigs of Aralia cachemirica and purified Continentalic acid isolated from this extract against a panel of human cancer cell lines of varied tissues. Percentage of growth inhibition was evaluated by sulphorhodamine B assay. Purified Continentalic acid showed moderate cytotoxicity against all the cell lines used. In contrast, the extract exhibited significant concentration dependant cytotoxicity against A-549 (lung), THP-1 (leukemia) and MCF-7 (breast) cell lines. This work highlights cytotoxic potential of this extract, which can further be explored for different constituents for their possible use autonomously or in combined manner in cancer therapy. The detailed analysis of their cytotoxicity has been presented in this paper.
Continentalic acid from Aralia continentalis induces growth inhibition and apoptosis in HepG2 cells.[Pubmed:18806961]
Arch Pharm Res. 2008 Sep;31(9):1172-8.
In this study, we investigated the effects of Continentalic acid (CA, (-)-pimara-8(14), 15-diene-19-oic acid), a diterpenic acid, isolated from Aralia continentalis, on the proliferation and apoptosis induction of HepG2 cells. In 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, the inhibitory effect became gradually stronger with the passage of time, 24, 48 and 72 h after treatment with CA, and the most significant effect was observed at 72 h. CA treatment for 72 h induced DNA fragmentation in a dose-dependent manner. Furthermore, flow cytometric analysis of HepG2 cells exposed to CA showed that apoptotic cells increased in a dose-dependent manner. The induction of apoptosis in HepG2 cells by CA was mediated through the activation of caspase-3, Bak, and Bax, and then through the cleavage of peroxisome proliferator-activated receptor (PARP) and the down-regulation of Bcl-2. These results demonstrate that CA efficiently induces apoptosis and is a good candidate for further evaluation as an effective chemotherapeutic agent.
Anti-inflammatory activity of the constituents of the roots of Aralia continentalis.[Pubmed:19784580]
Arch Pharm Res. 2009 Sep;32(9):1237-43.
To assess the anti-inflammatory activity of the constituents of the roots of Aralia continentalis, ent-pimara-8(14),15-diene-19-oic acid (Continentalic acid, pimaradienoic acid, compound I), 7beta-hydroxy-ent-pimara-8(14),15-diene-19-oic acid (compound II), 7-oxo-ent-pimara-8(14),15-diene-19-oic acid (compound III), 15alpha,16alpha-epoxy-17-hydroxy-ent-kauran-19-oic acid (compound IV) and ent-kaura-16-en-19-oic acid (kaurenoic acid, compound V), their inhibitory effects against cyclooxygenase-2 (COX-2)-catalyzed PGE(2) and inducible nitric oxide synthase (iNOS)-catalyzed NO production by lipopolysaccharide-treated RAW 264.7 cells were examined. Among the compounds tested, compound III and V moderately inhibited NO production. In addition, compound III weakly inhibited PGE2 production, while treatment with compounds II and IV at concentrations of up to 100 microM had no significant effects. Conversely, compound I only weakly inhibited PGE2 and NO production. To elucidate the mechanism by which these changes occurred, the iNOS down-regulating capacity of compound III was investigated. Western blot analysis and an electrophoretic mobility shift assay demonstrated that compound III weakly inhibited COX-2 and iNOS expression at 50-100 microM, and inhibited NF-kappaB activation. When in vivo anti-inflammatory activities of compounds I, III and V were examined, intraperitoneal injection of 4-100 mg/kg of compound I and V significantly inhibited carrageenan-induced paw edema in mice, whereas compound III did not. Taken together, the results of this study suggest that some constituents of A. continentalis, especially compounds I, III and V, exert significant anti-inflammatory activity, which suggests that these constituents contribute, at least in part, to the anti-inflammatory action of the roots of A. continentalis.


