Alisol ACAS# 19885-10-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19885-10-0 | SDF | Download SDF |
| PubChem ID | 15558616 | Appearance | White powder |
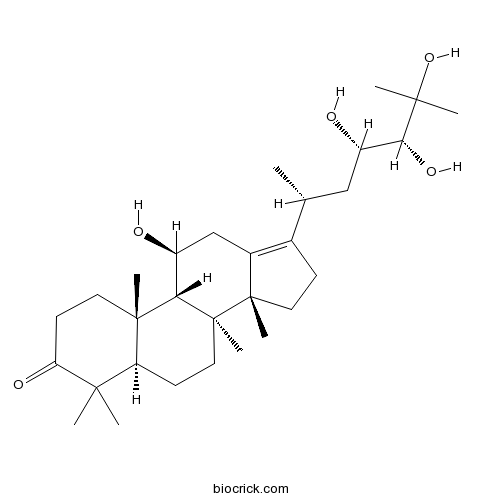
| Formula | C30H50O5 | M.Wt | 490.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Alisol-A | ||
| Solubility | Soluble in chloroform | ||
| Chemical Name | (5R,8S,9S,10S,11S,14R)-11-hydroxy-4,4,8,10,14-pentamethyl-17-[(2R,4S,5R)-4,5,6-trihydroxy-6-methylheptan-2-yl]-1,2,5,6,7,9,11,12,15,16-decahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CC(CC(C(C(C)(C)O)O)O)C1=C2CC(C3C4(CCC(=O)C(C4CCC3(C2(CC1)C)C)(C)C)C)O | ||
| Standard InChIKey | HNOSJVWYGXOFRP-UNPOXIGHSA-N | ||
| Standard InChI | InChI=1S/C30H50O5/c1-17(15-21(32)25(34)27(4,5)35)18-9-13-29(7)19(18)16-20(31)24-28(6)12-11-23(33)26(2,3)22(28)10-14-30(24,29)8/h17,20-22,24-25,31-32,34-35H,9-16H2,1-8H3/t17-,20+,21+,22+,24+,25-,28+,29+,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Alisol A presents inhibitory effects on cancer cell lines tested(HepG2, MDA-MB-231, and MCF-7 cells). 2. Alisol A enhances LC3II expression, indicates autophagy is occured, suggests that alisol A may be an autophagic inducer. |
| Targets | P450 (e.g. CYP17) | NO | Autophagy |

Alisol A Dilution Calculator

Alisol A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0379 mL | 10.1895 mL | 20.3791 mL | 40.7581 mL | 50.9476 mL |
| 5 mM | 0.4076 mL | 2.0379 mL | 4.0758 mL | 8.1516 mL | 10.1895 mL |
| 10 mM | 0.2038 mL | 1.019 mL | 2.0379 mL | 4.0758 mL | 5.0948 mL |
| 50 mM | 0.0408 mL | 0.2038 mL | 0.4076 mL | 0.8152 mL | 1.019 mL |
| 100 mM | 0.0204 mL | 0.1019 mL | 0.2038 mL | 0.4076 mL | 0.5095 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Alisol A is a natural product.
References:
[1]. Yu Y, et al.In vitro metabolism of alisol A and its metabolites' identification using high-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Dec 15;941:31-7.
[2]. Yu Y, et al. A sensitive liquid chromatography-mass spectrometry method for simultaneous determination of alisol A and alisol A 24-acetate from Alisma orientale (Sam.) Juz. in rat plasma. Anal Bioanal Chem. 2011 Jan;399(3):1363-9
- H-Phe(2-F)-OH
Catalog No.:BCC3222
CAS No.:19883-78-4
- H-D-Phg-OMe.HCl
Catalog No.:BCC3314
CAS No.:19883-41-1
- Z-D-Tyr(tBu)-OH.DCHA
Catalog No.:BCC2744
CAS No.:198828-72-7
- (7R)-Methoxy-8-epi-matairesinol
Catalog No.:BCN7582
CAS No.:198827-23-5
- Merimepodib
Catalog No.:BCC4128
CAS No.:198821-22-6
- Windorphen
Catalog No.:BCC6486
CAS No.:19881-70-0
- Bavachin
Catalog No.:BCN4872
CAS No.:19879-32-4
- Bavachinin
Catalog No.:BCN4871
CAS No.:19879-30-2
- 1,3-Dicaffeoylquinic acid
Catalog No.:BCN2972
CAS No.:19870-46-3
- MLCK inhibitor peptide
Catalog No.:BCC5852
CAS No.:198694-74-5
- Cabralealactone
Catalog No.:BCN4870
CAS No.:19865-87-3
- Alisol B acetate
Catalog No.:BCN2304
CAS No.:19865-76-0
- Humulene epoxide II
Catalog No.:BCN4873
CAS No.:19888-34-7
- Continentalic acid
Catalog No.:BCN6526
CAS No.:19889-23-7
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Nagilactone B
Catalog No.:BCN4049
CAS No.:19891-51-1
- Jaborosalactone D
Catalog No.:BCN7946
CAS No.:19891-82-8
- 29-Nor-20-oxolupeol
Catalog No.:BCN6678
CAS No.:19891-85-1
- Kaempferol 3-O-beta-sophoroside
Catalog No.:BCN3336
CAS No.:19895-95-5
- Syringaresinol diacetate
Catalog No.:BCN4874
CAS No.:1990-77-8
- Dihydromethysticin
Catalog No.:BCN2476
CAS No.:19902-91-1
- Bakkenolide A
Catalog No.:BCN5402
CAS No.:19906-72-0
- Maackiain
Catalog No.:BCN1236
CAS No.:19908-48-6
- H-Phe(4-Me)-OH
Catalog No.:BCC3270
CAS No.:1991-87-3
Structures and biological activities of the triterpenoids and sesquiterpenoids from Alisma orientale.[Pubmed:27615692]
Phytochemistry. 2016 Nov;131:150-157.
Sixteen triterpenoids and nine sesquiterpenoids were isolated from the rhizome of Alisma orientale. Structures of 16-oxo-11-anhydroAlisol A 24-acetate, 13beta,17beta-epoxy-24,25,26,27-tetranor-Alisol A 23-oic acid, 1alphaH,5alphaH-guaia-6-ene-4beta,10beta-diol, and alisguaiaone were elucidated by comprehensive spectroscopic data analysis. The cytotoxic, antibacterial, antifungal, anti-inflammatory, and alpha-glucosidase inhibitory activities of isolated terpenoids were evaluated. Triterpenoids Alisol A, Alisol A 24-acetate, 25-O-ethylAlisol A, 11-deoxyAlisol A, alisol E 24-acetate, alisol G, alisol B 23-acetate and sesquiterpenoids 1alphaH,5alphaH-guaia-6-ene-4beta,10beta-diol, 10-hydroxy-7,10-epoxysalvialane exhibited cytotoxicities against the three tested human cancer cell lines with IC50 values ranging from 11.5 +/- 1.7 muM to 76.7 +/- 1.4 muM. Triterpenoids Alisol A, 25-O-ethylAlisol A, 11-deoxyAlisol A, alisol E 24-acetate, alisol G, and 25-anhydroalisol F showed antibacterial activities against the Gram-positive strains Bacillus subtilis and Staphylococcus aureus with MIC values of 12.5-100 mug/mL. Sesquiterpenoid 4beta,10beta-dihydroxy-1alphaH,5betaH-guaia-6-ene exhibited antibacterial activity against B. subtilis with an MIC value of 50 mug/mL, and 10-hydroxy-7,10-epoxysalvialane exhibited activity against S. aureus with an MIC value of 100 mug/mL. Compounds 16-oxo-11-anhydroAlisol A 24-acetate, alisol F, 25-anhydroalisol F, and alisguaiaone exhibited inhibitory effects on lipopolysaccharide-induced NO production in RAW 264.7 macrophage cells. None of the compounds showed obvious inhibitory activity against alpha-glucosidase.
Anti-proliferative activities of terpenoids isolated from Alisma orientalis and their structure-activity relationships.[Pubmed:24893804]
Anticancer Agents Med Chem. 2015;15(2):228-35.
This study aimed to isolate terpenoids from Alisma orientalis (Sam.) Juzep. and elucidate their antiproliferative activities, as well as structure-activity relationships. Fourteen protostane-type triterpenoids were isolated from the rhizome of A. orientalis. Among these triterpenoids, Alisol A (1), Alisol A 24-acetate (2), alisol B (3), alisol B 23-acetate (4), and alisol G (8) presented inhibitory effects on cancer cell lines tested. Compounds 3 and 4 showed the highest potential; IC50 values for HepG2, MDA-MB-231, and MCF-7 cells were 16.28, 14.47, and 6.66 muM for 3 and 18.01, 15.97, and 13.56 muM for 4, respectively. Based on these results, we concluded that the degree of C-16 oxidation and the double bond between C-13 and C-17 may be significant in anti-proliferative activities. Further study showed that 3 and 4 effectively induced apoptosis, as confirmed by flow cytometry. Increased intracellular calcium concentration and endoplasmic reticulum stress were detected after treatment with 4 in HepG2 cells. Although compounds 1 and 2 induced minimal apoptosis, they evidently delayed the G2/M phase in HepG2 cells. Further study showed that 1-4 also enhanced LC3II expression, indicating autophagy is occured.


