MerimepodibCAS# 198821-22-6 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 198821-22-6 | SDF | Download SDF |
| PubChem ID | 153241 | Appearance | Powder |
| Formula | C23H24N4O6 | M.Wt | 452.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | VI-21497; VX-497; MMP | ||
| Solubility | >45.2mg/mL in DMSO | ||
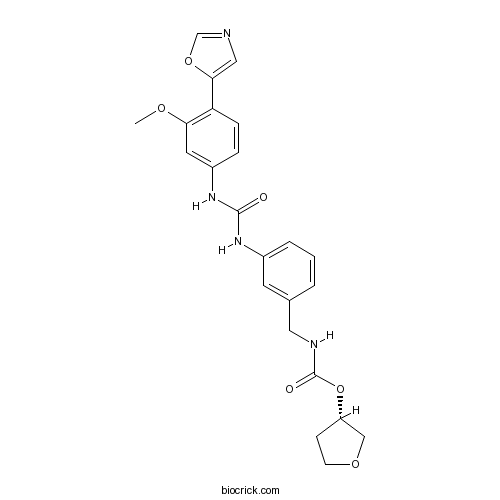
| Chemical Name | [(3S)-oxolan-3-yl] N-[[3-[[3-methoxy-4-(1,3-oxazol-5-yl)phenyl]carbamoylamino]phenyl]methyl]carbamate | ||
| SMILES | COC1=C(C=CC(=C1)NC(=O)NC2=CC=CC(=C2)CNC(=O)OC3CCOC3)C4=CN=CO4 | ||
| Standard InChIKey | JBPUGFODGPKTDW-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C23H24N4O6/c1-30-20-10-17(5-6-19(20)21-12-24-14-32-21)27-22(28)26-16-4-2-3-15(9-16)11-25-23(29)33-18-7-8-31-13-18/h2-6,9-10,12,14,18H,7-8,11,13H2,1H3,(H,25,29)(H2,26,27,28)/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Merimepodib is a novel noncompetitive inhibitor of IMPDH (Inosine monophosphate dehydrogenase).In Vitro:VX-497 has antiproliferative effect on lymphoid and keratinocyte cells. The antiproliferative effect of VX-497 in cells is reversed within 48 h of its removal[1]. VX-497 has intermediate antiviral activity against a second group of viruses, which includes HSV-1, parainfluenza-3 virus, BVDV, VEEV, and dengue virus, with IC50s ranging from 6 to 19 μM. VX-497 is 100-fold more potent, with an IC50 of 380 nM and a corresponding CC50 of 5.2 μM, for a therapeutic index of 14. The antiviral activity of VX-497 in HepG2.2.2.15 cells is reversed threefold by the addition of guanosine[2].In Vivo:Oral administration of VX-497 inhibits the primary IgM antibody response in a dose-dependent manner, with an ED50 value of appr 30-35 mg/kg in mice. Single daily dosing of VX-497 is observed to be as effective as twice-daily dosing in this model of immune activation[1]. GVHD developed in the vehicle-treated allografted F1 mice and treatment with VX-497 improved all manifestations of the disease significantly. The 2.9-fold increase in spleen weight in allografted animals is reduced to a 1.6-fold increase in the VX-497-treated mice. Serum IFN-gamma levels are increased 54-fold in the vehicle group while there is a 7.4-fold increase in VX-497-treated animals[3]. References: | |||||

Merimepodib Dilution Calculator

Merimepodib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2101 mL | 11.0507 mL | 22.1014 mL | 44.2028 mL | 55.2535 mL |
| 5 mM | 0.442 mL | 2.2101 mL | 4.4203 mL | 8.8406 mL | 11.0507 mL |
| 10 mM | 0.221 mL | 1.1051 mL | 2.2101 mL | 4.4203 mL | 5.5254 mL |
| 50 mM | 0.0442 mL | 0.221 mL | 0.442 mL | 0.8841 mL | 1.1051 mL |
| 100 mM | 0.0221 mL | 0.1105 mL | 0.221 mL | 0.442 mL | 0.5525 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Merimepodiba(VX-497) is a novel noncompetitive inhibitor of IMPDH(Inosine monophosphate dehydrogenase).
- Windorphen
Catalog No.:BCC6486
CAS No.:19881-70-0
- Bavachin
Catalog No.:BCN4872
CAS No.:19879-32-4
- Bavachinin
Catalog No.:BCN4871
CAS No.:19879-30-2
- 1,3-Dicaffeoylquinic acid
Catalog No.:BCN2972
CAS No.:19870-46-3
- MLCK inhibitor peptide
Catalog No.:BCC5852
CAS No.:198694-74-5
- Cabralealactone
Catalog No.:BCN4870
CAS No.:19865-87-3
- Alisol B acetate
Catalog No.:BCN2304
CAS No.:19865-76-0
- Alisol A 23-acetate
Catalog No.:BCN3457
CAS No.:19865-75-9
- Tranylcypromine hydrochloride
Catalog No.:BCC7791
CAS No.:1986-47-6
- Fmoc-Ser(tBu)-ol
Catalog No.:BCC2578
CAS No.:198561-87-4
- Fmoc-HoTyr-OH.DCHA
Catalog No.:BCC3246
CAS No.:198560-10-0
- Fmoc-Asparaginol(Trt)
Catalog No.:BCC3042
CAS No.:198543-08-7
- (7R)-Methoxy-8-epi-matairesinol
Catalog No.:BCN7582
CAS No.:198827-23-5
- Z-D-Tyr(tBu)-OH.DCHA
Catalog No.:BCC2744
CAS No.:198828-72-7
- H-D-Phg-OMe.HCl
Catalog No.:BCC3314
CAS No.:19883-41-1
- H-Phe(2-F)-OH
Catalog No.:BCC3222
CAS No.:19883-78-4
- Alisol A
Catalog No.:BCN3455
CAS No.:19885-10-0
- Humulene epoxide II
Catalog No.:BCN4873
CAS No.:19888-34-7
- Continentalic acid
Catalog No.:BCN6526
CAS No.:19889-23-7
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Nagilactone B
Catalog No.:BCN4049
CAS No.:19891-51-1
- Jaborosalactone D
Catalog No.:BCN7946
CAS No.:19891-82-8
- 29-Nor-20-oxolupeol
Catalog No.:BCN6678
CAS No.:19891-85-1
- Kaempferol 3-O-beta-sophoroside
Catalog No.:BCN3336
CAS No.:19895-95-5
Merimepodib, pegylated interferon, and ribavirin in genotype 1 chronic hepatitis C pegylated interferon and ribavirin nonresponders.[Pubmed:19852040]
Hepatology. 2009 Dec;50(6):1719-26.
UNLABELLED: Merimepodib (MMPD) is an orally administered, inosine monophosphate dehydrogenase inhibitor that has shown antiviral activity in nonresponders with chronic hepatitis C (CHC) when combined with pegylated interferon alfa 2a (Peg-IFN-alfa-2a) and ribavirin (RBV). We conducted a randomized, double-blind, multicenter, phase 2b study to evaluate the antiviral activity, safety, and tolerability of MMPD in combination with Peg-IFN-alfa-2a and RBV in patients with genotype 1 CHC who were nonresponders to prior therapy with Peg-IFN and RBV. Patients received 50 mg MMPD, 100 mg MMPD, or placebo every 12 hours, in addition to Peg-IFN-alfa-2a and RBV, for 24 weeks. Patients with a 2-log or more decrease from baseline or undetectable hepatitis C virus (HCV) RNA levels at week 24 were then eligible to continue Peg-IFN-alfa-2a and RBV for a further 24 weeks, followed by 24 weeks of follow-up. The primary efficacy endpoint was sustained virological response (SVR) rate at week 72 in all randomized patients who received at least one dose of study drug and had a history of nonresponse to standard therapy. A total of 354 patients were randomized to treatment (117 to placebo; 119 to 50 mg MMPD; 118 to 100 mg MMPD), and 286 completed the core study. The proportion of patients who achieved SVR was similar among the treatment groups: 6% (6/107) for 50 mg MMPD, 4% (5/112) for 100 mg MMPD, and 5% (5/104) for placebo (P = 0.8431). Adverse-event profiles for the MMPD combination groups were similar to that for Peg-IFN-alfa and RBV alone. Nausea, arthralgia, cough, dyspnea, neutropenia, and anemia were more common in patients taking MMPD. CONCLUSION: The addition of MMPD to Peg-IFN-alfa-2a and RBV combination therapy did not increase the proportion of nonresponder patients with genotype 1 CHC achieving an SVR.
A randomized, double-blind, placebo-controlled dose-escalation trial of merimepodib (VX-497) and interferon-alpha in previously untreated patients with chronic hepatitis C.[Pubmed:16152757]
Antivir Ther. 2005;10(5):635-43.
Inhibition of inosine monophosphate dehydrogenase (IMPDH) is one of several proposed mechanisms of action for ribavirin (RBV), a critical component of the current treatment for chronic hepatitis C (CHC). This study was a double-blind, placebo-controlled dose-escalation study of a novel, selective, orally active small molecule inhibitor of IMPDH, Merimepodib (VX-497 or MMPD) in combination with standard interferon-alpha (IFN-alpha). Fifty-four treatment-naive patients with genotype-1 CHC were randomized to receive IFN-alpha 3 MIU subcutaneously three times a week, alone or in combination with 100 mg or 300 mg (every 8 h) of MMPD for 4 weeks. At the end of 4 weeks, all patients were offered 48 weeks of treatment with IFN-alpha/RBV. The objectives of the study were to evaluate the tolerability of the IFN-alpha/MMPD combination and to evaluate whether MMPD had an on-treatment effect on HCV-RNA, similar to RBV when added to IFN-alpha. The drug combination was generally well tolerated; one patient at the higher dose discontinued because of elevated alanine aminotransferase levels. No pharmacokinetic interactions were evident between the two drugs. Analysis of covariance that adjusted for a baseline imbalance in HCV-RNA in the intent-to-treat population did not show any significant differences between the treatment groups, or between MMPD plus IFN-alpha compared with IFN-alpha alone. However, the per-protocol primary efficacy analysis based on treatment-compliant patients demonstrated a greater reduction in mean HCV-RNA in the combination of 100 mg MMPD plus IFN-alpha compared with IFN-alpha alone (-1.78 log vs -0.86 log, P=0.037). In conclusion, the addition of a selective IMPDH inhibitor to IFN-alpha was well tolerated. In a low-dose range, the addition of MMPD may have the potential to add to the antiviral efficacy of IFN-alpha. Larger, longer duration trials incorporating pegylated IFN would be required to determine whether this combination, alone or with RBV, would increase either early or sustained virological response rates.
Phase 2 study of the combination of merimepodib with peginterferon-alpha2b, and ribavirin in nonresponders to previous therapy for chronic hepatitis C.[Pubmed:17629590]
J Hepatol. 2007 Oct;47(4):476-83.
BACKGROUND/AIMS: While combination of peginterferon-alpha (PEG-IFN) and ribavirin (RBV) therapy is the current standard of care for chronic hepatitis C (CHC), only 44-51% of genotype-1 patients achieve a sustained virological response (SVR), and both agents produce treatment-limiting toxicities. In the hepatitis C virus (HCV) replicon system, Merimepodib (MMPD), a novel, selective inhibitor of inosine monophosphate dehydrogenase, has shown potent antiviral effects. METHODS: This randomized, placebo-controlled, double-blind study evaluated the safety and antiviral activity of PEG-IFN-alpha2b and RBV combined with either placebo, 25mg MMPD every 12h (q12h), or 50mg MMPD q12h in interferon-alpha (IFN) and RBV nonresponders. After 24 weeks of treatment, subjects with undetectable HCV RNA were proposed to continue assigned treatment for up to 24 additional weeks. RESULTS: The PEG-IFN-alpha, RBV, and MMPD combination was well tolerated at both doses. After 24 weeks, the proportion of HCV RNA undetectable subjects was 8/11 (73%) in the 50-mg MMPD group, 2/10 (20%) in the 25-mg MMPD group, and 3/10 (30%) in the placebo group (P=0.02, Jonckheere-Terpstra test for increasing dose response). Ten subjects entered and completed an extension study, at Week 48, 2 of 2 (100%) of the 25-mg and 3 of 5 (60%) of the 50-mg subjects remained HCV RNA undetectable, compared with 3 of 3 (100%) of the placebo subjects. At Follow-up Week 24, 2 (100%) of the 25-mg , and 1 (25%) of the 50-mg subjects remained undetectable, compared with 1 (33%) of the placebo subjects. Pharmacokinetic and pharmacodynamic analyses showed a correlation between MMPD exposure and early virological response at week 12, but not with hemoglobin decreases often associated with RBV. CONCLUSIONS: In conclusion, PEG-IFN-alpha2b and RBV combined with 50 mg MMPD q12h was well tolerated and induced virological response with undetectable HCV RNA in IFN-alpha and RBV nonresponders.


