BavachinCAS# 19879-32-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19879-32-4 | SDF | Download SDF |
| PubChem ID | 14236566 | Appearance | White powder |
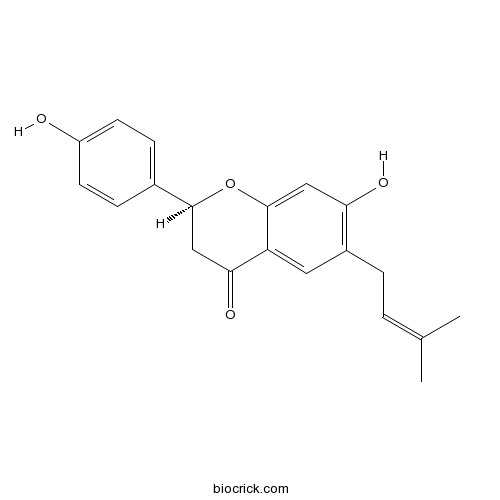
| Formula | C20H20O4 | M.Wt | 324.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Corylifolin; 4',7-Dihydroxy 6-(3-methyl 2-butenyl)flavanone | ||
| Solubility | DMSO : ≥ 150 mg/mL (462.43 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S)-7-hydroxy-2-(4-hydroxyphenyl)-6-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1)C(=O)CC(O2)C3=CC=C(C=C3)O)O)C | ||
| Standard InChIKey | OAUREGNZECGNQS-IBGZPJMESA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bavachin is a phytoestrogen that activates the estrogen receptors ERα and ERβ with EC50s of 320 and 680 nM, respectively. It is a cholesterol acyltransferase inhibitor, may have therapeutic potential for type 2 diabetes by activating insulin signaling pathways. Bavachin can stimulate the genetic expression of VEGF in PB,and directly help the fracture healing, and potentially protect cartilage from inflammation-mediated damage in joints of osteoarthritis patients through decreasing IL-1β-induced activation of IKK-IκBα-NF-κB signaling pathway. Bavachin has suppressive effects against pigmentation by melanin in the skin. |
| Targets | NF-kB | p65 | IkB | VEGFR | IL Receptor | AP-1 | AMPK | PPAR | GLUT | Akt | IKK | Estrogen receptor α | Estrogen receptor β |
| In vivo | Bavachin from Psoralea corylifolia Improves Insulin-Dependent Glucose Uptake through Insulin Signaling and AMPK Activation in 3T3-L1 Adipocytes.[Pubmed: 27070585]Int J Mol Sci. 2016 Apr 8;17(4):527.The fruit of Psoralea corylifolia L. (Fabaceae) (PC), known as "Bo-Gol-Zhee" in Korea has been used as traditional medicine. Ethanol and aqueous extracts of PC have an anti-hyperglycemic effect by increasing plasma insulin levels and decreasing blood glucose and total plasma cholesterol levels in type 2 diabetic rats.
Effect of Brain Injury and Bavachin on 5-HT and VEGF of Rats with Tibial Fracture.[Reference: WebLink]Journal of Emergency in Traditional Chinese Medicine, 2014, 23(9):1585-8.To observe the influence of brain injury and Bavachin on 5-HT and VEGF during the healing of rats′ tibial fracture.
|
| Kinase Assay | Activation of Estrogen Receptor by Bavachin from Psoralea corylifolia.[Pubmed: 24116293]Biomol Ther (Seoul). 2012 Mar;20(2):183-8.In this study, we examined the estrogenic activity of Bavachin, a component of Psoralea corylifolia that has been used as a traditional medicine in Asia.
|
| Cell Research | Phytoestrogen bavachin mediates anti-inflammation targeting Ikappa B kinase-I kappaB alpha-NF-kappaB signaling pathway in chondrocytes in vitro.[Pubmed: 20361957]Eur J Pharmacol. 2010 Jun 25;636(1-3):181-8.The pro-inflammatory cytokine interleukin-1 beta (IL-1 beta) plays critical roles in pathogenesis of osteoarthritis. Although estrogen is protective for cartilage in osteoarthritis patients, it also potentially increases the risk of stroke and cancer. Phytoestrogens acting as natural estrogen receptor modulators may serve as alternatives.
|
| Animal Research | Inhibitory effects of bakuchiol, bavachin, and isobavachalcone isolated from Piper longum on melanin production in B16 mouse melanoma cells.[Pubmed: 20622433]Biosci Biotechnol Biochem. 2010;74(7):1504-6.
|
| Structure Identification | Arch Pharm Res. 2008 Nov;31(11):1419-23.Bavachin and isobavachalcone, acyl-coenzyme A: cholesterol acyltransferase inhibitors from Psoralea corylifolia.[Pubmed: 19023538]Acyl-coenzyme A: cholesterol acyltransferase (ACAT) catalyzes cholesterol esterification and plays important roles in intestinal absorption of cholesterol, hepatic production of lipoproteins and accumulation of cholesteryl ester within macrophages and smooth muscle cells.
|

Bavachin Dilution Calculator

Bavachin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0826 mL | 15.4131 mL | 30.8261 mL | 61.6523 mL | 77.0654 mL |
| 5 mM | 0.6165 mL | 3.0826 mL | 6.1652 mL | 12.3305 mL | 15.4131 mL |
| 10 mM | 0.3083 mL | 1.5413 mL | 3.0826 mL | 6.1652 mL | 7.7065 mL |
| 50 mM | 0.0617 mL | 0.3083 mL | 0.6165 mL | 1.233 mL | 1.5413 mL |
| 100 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6165 mL | 0.7707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bavachinin
Catalog No.:BCN4871
CAS No.:19879-30-2
- 1,3-Dicaffeoylquinic acid
Catalog No.:BCN2972
CAS No.:19870-46-3
- MLCK inhibitor peptide
Catalog No.:BCC5852
CAS No.:198694-74-5
- Cabralealactone
Catalog No.:BCN4870
CAS No.:19865-87-3
- Alisol B acetate
Catalog No.:BCN2304
CAS No.:19865-76-0
- Alisol A 23-acetate
Catalog No.:BCN3457
CAS No.:19865-75-9
- Tranylcypromine hydrochloride
Catalog No.:BCC7791
CAS No.:1986-47-6
- Fmoc-Ser(tBu)-ol
Catalog No.:BCC2578
CAS No.:198561-87-4
- Fmoc-HoTyr-OH.DCHA
Catalog No.:BCC3246
CAS No.:198560-10-0
- Fmoc-Asparaginol(Trt)
Catalog No.:BCC3042
CAS No.:198543-08-7
- 9,10-Anthracenedione
Catalog No.:BCN3469
CAS No.:19852-76-7
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- Windorphen
Catalog No.:BCC6486
CAS No.:19881-70-0
- Merimepodib
Catalog No.:BCC4128
CAS No.:198821-22-6
- (7R)-Methoxy-8-epi-matairesinol
Catalog No.:BCN7582
CAS No.:198827-23-5
- Z-D-Tyr(tBu)-OH.DCHA
Catalog No.:BCC2744
CAS No.:198828-72-7
- H-D-Phg-OMe.HCl
Catalog No.:BCC3314
CAS No.:19883-41-1
- H-Phe(2-F)-OH
Catalog No.:BCC3222
CAS No.:19883-78-4
- Alisol A
Catalog No.:BCN3455
CAS No.:19885-10-0
- Humulene epoxide II
Catalog No.:BCN4873
CAS No.:19888-34-7
- Continentalic acid
Catalog No.:BCN6526
CAS No.:19889-23-7
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Nagilactone B
Catalog No.:BCN4049
CAS No.:19891-51-1
- Jaborosalactone D
Catalog No.:BCN7946
CAS No.:19891-82-8
Phytoestrogen bavachin mediates anti-inflammation targeting Ikappa B kinase-I kappaB alpha-NF-kappaB signaling pathway in chondrocytes in vitro.[Pubmed:20361957]
Eur J Pharmacol. 2010 Jun 25;636(1-3):181-8.
The pro-inflammatory cytokine interleukin-1 beta (IL-1 beta) plays critical roles in pathogenesis of osteoarthritis. Although estrogen is protective for cartilage in osteoarthritis patients, it also potentially increases the risk of stroke and cancer. Phytoestrogens acting as natural estrogen receptor modulators may serve as alternatives. This study aimed to identify medicinal phytoestrogens that preserve anti-inflammatory property and may function as potential chondro-protective compounds. Both human chondrocytes and chondrocytic cell line CHON-002 were used for this study. Protein concentrations or expressions were measured by ELISA or Western blot, respectively. The DNA-binding activity and transcriptional activity of transcription factors were evaluated by electrophoretic mobility shift assay and dual-luciferase reporter assay, respectively. Cell migration was analyzed by chemotaxis assays. We found that among screened phytoestrogens, Bavachin could potently decrease IL-1 beta-induced nuclear factor-kappa B (NF-kappaB) but not activator protein-1 (AP-1) DNA-binding activity. Bavachin also inhibited I kappaB alpha degradation, increased nuclear translocation of p65 and p50 as well as decreased I kappaB alpha kinase (IKK) activity. Furthermore, Bavachin inhibited IL-1 beta-induced chemokine production that resulted in reduced migration of THP-1 monocytic cells. Our results suggest that through decreasing IL-1 beta-induced activation of IKK-I kappaB alpha-NF-kappaB signaling pathway, Bavachin potentially protects cartilage from inflammation-mediated damage in joints of osteoarthritis patients.
Inhibitory effects of bakuchiol, bavachin, and isobavachalcone isolated from Piper longum on melanin production in B16 mouse melanoma cells.[Pubmed:20622433]
Biosci Biotechnol Biochem. 2010;74(7):1504-6.
An EtOH extract of fruits of Piper longum was found to exhibit a potent inhibitory effect against alpha-melanocyte-stimulating hormone (alpha-MSH)-induced melanin production in B16 mouse melanoma cells. Bioassay-directed fractionation led to the isolation of prenylated phenolic compounds bakuchiol, Bavachin, and isobavachalcone. These compounds and the crude extract of the fruits of P. longum may have suppressive effects against pigmentation by melanin in the skin.
Bavachin from Psoralea corylifolia Improves Insulin-Dependent Glucose Uptake through Insulin Signaling and AMPK Activation in 3T3-L1 Adipocytes.[Pubmed:27070585]
Int J Mol Sci. 2016 Apr 8;17(4):527.
The fruit of Psoralea corylifolia L. (Fabaceae) (PC), known as "Bo-Gol-Zhee" in Korea has been used as traditional medicine. Ethanol and aqueous extracts of PC have an anti-hyperglycemic effect by increasing plasma insulin levels and decreasing blood glucose and total plasma cholesterol levels in type 2 diabetic rats. In this study, we purified six compounds from PC and investigated their anti-diabetic effect. Among the purified compounds, Bavachin most potently accumulated lipids during adipocyte differentiation. Intracellular lipid accumulation was measured by Oil Red-O (ORO) cell staining to investigate the effect of compounds on adipogenesis. Consistently, Bavachin activated gene expression of adipogenic transcriptional factors, proliferator-activated receptorgamma (PPARgamma) and CCAAT/enhancer binding protein-alpha (C/EBPalpha). Bavachin also increased adiponectin expression and secretion in adipocytes. Moreover, Bavachin increased insulin-induced glucose uptake by differentiated adipocytes and myoblasts. In differentiated adipocytes, we found that Bavachin enhanced glucose uptake via glucose transporter 4 (GLUT4) translocation by activating the Akt and 5'AMP-activated protein kinase (AMPK) pathway in the presence or absence of insulin. These results suggest that Bavachin from Psoralea corylifolia might have therapeutic potential for type 2 diabetes by activating insulin signaling pathways.
Bavachin and isobavachalcone, acyl-coenzyme A: cholesterol acyltransferase inhibitors from Psoralea corylifolia.[Pubmed:19023538]
Arch Pharm Res. 2008 Nov;31(11):1419-23.
Acyl-coenzyme A: cholesterol acyltransferase (ACAT) catalyzes cholesterol esterification and plays important roles in intestinal absorption of cholesterol, hepatic production of lipoproteins and accumulation of cholesteryl ester within macrophages and smooth muscle cells. Ethanol extract of Psoralea corylifolia showed a significant inhibition of ACAT enzyme. Via bioactivity-guided fractionation of the ethanol extract of Psoralea corylifolia, two prenylated flavonoids were isolated. Their structures were determined as Bavachin (1) and isobavachalcone (2) by spectroscopic analysis ((1)H-, (13)C-NMR, 2DNMR, and ESI-MS). The IC(50) values were 86.0 (1) and 48.0 (2) microM in the ACAT assay system using rat liver microsome. Compound 2 also decreased cholesteryl ester formations in HepG2 cells. In addition, this compound showed a noncompetitive type of inhibition of ACAT.
Activation of Estrogen Receptor by Bavachin from Psoralea corylifolia.[Pubmed:24116293]
Biomol Ther (Seoul). 2012 Mar;20(2):183-8.
In this study, we examined the estrogenic activity of Bavachin, a component of Psoralea corylifolia that has been used as a traditional medicine in Asia. Bavachin was purified from ethanolic extract of Psoralea corylifolia and characterized its estrogenic activity by ligand binding, reporter gene activation, and endogenous estrogen receptor (ER) target gene regulation. Bavachin showed ER ligand binding activity in competitive displacement of [(3)H] E2 from recombinant ER. The estrogenic activity of Bavachin was characterized in a transient transfection system using ERalpha or ERbeta and estrogen-responsive luciferase plasmids in CV-1 cells with an EC50 of 320 nM and 680 nM, respectively. Bavachin increased the mRNA levels of estrogen-responsive genes such as pS2 and PR, and decreased the protein level of ERalpha by proteasomal pathway. However, Bavachin failed to activate the androgen receptor in CV-1 cells transiently transfected with the corresponding receptor and hormone responsive reporter plasmid. These data indicate that Bavachin acts as a weak phytoestrogen by binding and activating the ER.


