BavachininCAS# 19879-30-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19879-30-2 | SDF | Download SDF |
| PubChem ID | 10337211 | Appearance | Yellowish powder |
| Formula | C21H22O4 | M.Wt | 338.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 7-O-Methylbavachin; Bavachinin A | ||
| Solubility | DMSO : ≥ 250 mg/mL (738.77 mM) *"≥" means soluble, but saturation unknown. | ||
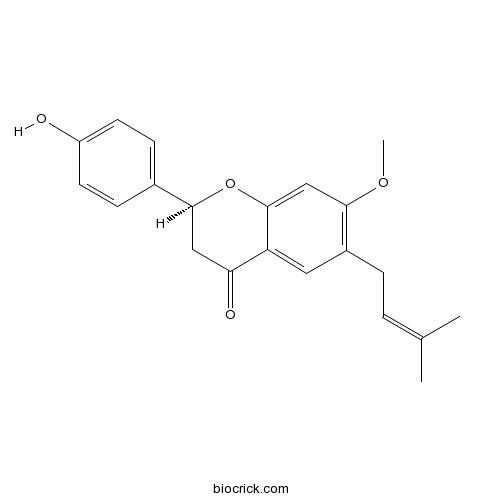
| Chemical Name | (2S)-2-(4-hydroxyphenyl)-7-methoxy-6-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1)C(=O)CC(O2)C3=CC=C(C=C3)O)OC)C | ||
| Standard InChIKey | VOCGSQHKPZSIKB-FQEVSTJZSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bavachinin is a novel natural pan-PPAR agonist , it shows stronger activities with PPAR-γ than with PPAR-α and PPAR-β/δ (EC50 = 0.74 μmol/l, 4.00 μmol/l and 8.07 μmol/l in 293T cells, respectively). Bavachinin possesses anti-asthma, anti-angiogenic , anti-inflammatory, antipyretic and analgesic properties, it also exhibits glucose-lowering properties without inducing weight gain and hepatotoxicity. |
| Targets | PPAR | HIF | VEGFR | GLUT | Immunology & Inflammation related |
| In vitro | Antiinflamatory, antipyretic & analgesic properties of bavachinin-a flavanone isolated from seeds of Psoralea corylifolia Linn. (Babchi).[Pubmed: 312274]Indian J. Exp. Biol., 1978, 16(11):1216-7.Antiinflamatory, antipyretic & analgesic properties of Bavachinin-a flavanone isolated from seeds of Psoralea corylifolia Linn. (Babchi). |
| In vivo | Anti-angiogenic and anti-tumor activity of Bavachinin by targeting hypoxia-inducible factor-1α.[Pubmed: 22760073]Eur J Pharmacol. 2012 Sep 15;691(1-3):28-37.Hypoxia-inducible factor-1 (HIF-1) consists of two subunits, the HIF-1β, which is constitutively expressed, and HIF-1α, which is oxygen-responsive. HIF-1α is over-expressed in response to hypoxia, increasing transcriptional activity linked to tumor progression, angiogenesis, metastasis, and invasion. This study aimed to demonstrate that the natural compound, Bavachinin, has potent anti-angiogenic activity in vitro and in vivo.
|
| Kinase Assay | Bavachinin, as a novel natural pan-PPAR agonist, exhibits unique synergistic effects with synthetic PPAR-γ and PPAR-α agonists on carbohydrate and lipid metabolism in db/db and diet-induced obese mice.[Pubmed: 26983922]Diabetologia. 2016 Jun;59(6):1276-86.
|
| Animal Research | Effect of Bavachinin and its derivatives on T cell differentiation.[Pubmed: 24508059]Int Immunopharmacol. 2014 Apr;19(2):399-404.Bavachinin, which can be isolated from the Chinese herb Fructus Psoraleae, has the potential as a potent anti-asthma drug. However, the extremely low water solubility of Bavachinin limits its application.
|
| Structure Identification | Bioorg Med Chem Lett. 2015 Jun 15;25(12):2579-83.Separation and peroxisome proliferator-activated receptor-γ agonist activity evaluation of synthetic racemic bavachinin enantiomers.[Pubmed: 25978962 ]Bavachinin, isolated from Psoralea corylifolia seeds, has been reported to demonstrate peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist activity. However, isolated Bavachinin is actually a mixture of S and R configurations, with an enantiomeric excess value of approximately 24.3%. For further study on the structure-activity relationships of Bavachinin, investigating the PPAR-γ agonist activity of the two enantiomers is crucial. |

Bavachinin Dilution Calculator

Bavachinin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9551 mL | 14.7754 mL | 29.5508 mL | 59.1017 mL | 73.8771 mL |
| 5 mM | 0.591 mL | 2.9551 mL | 5.9102 mL | 11.8203 mL | 14.7754 mL |
| 10 mM | 0.2955 mL | 1.4775 mL | 2.9551 mL | 5.9102 mL | 7.3877 mL |
| 50 mM | 0.0591 mL | 0.2955 mL | 0.591 mL | 1.182 mL | 1.4775 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2955 mL | 0.591 mL | 0.7388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bavachinin(7-O-Methylbavachin) is a natural compound isolated from the Chinese herb Fructus Psoraleae;has potent anti-angiogenic activity. IC50 value: Target: in vitro: Isobavachalcone significantly inhibits both oligomerization and fibrillization of Aβ42, whereas bavachinin inhibits fibrillization and leads to off-pathway aggregation. Both of the compounds attenuated Aβ42-induced toxicity in a SH-SY5Y cell model [1]. Bavachinin, has potent anti-angiogenic activity in vitro and in vivo. Bavachinin inhibited increases in HIF-1α activity in human KB carcinoma (HeLa cell derivative) and human HOS osteosarcoma cells under hypoxia in a concentration-dependent manner, probably by enhancing the interaction between von Hippel-Lindau (VHL) and HIF-1α [2]. in vivo: significantly inhibited Th2 cytokine production, including IL-4, IL-5 and IL-13. Notably, this compound almost completely blocked inflammation in the ovalbumin (OVA)-sensitized animal asthma model [3].
References:
[1]. Chen X, et al. Isobavachalcone and bavachinin from Psoraleae Fructus modulate Aβ42 aggregation process through different mechanisms in vitro. FEBS Lett. 2013 Sep 17;587(18):2930-5.
[2]. Nepal M, et al. Anti-angiogenic and anti-tumor activity of Bavachinin by targeting hypoxia-inducible factor-1α. Eur J Pharmacol. 2012 Sep 15;691(1-3):28-37.
[3]. Chen X, et al. Treatment of allergic inflammation and hyperresponsiveness by a simple compound, Bavachinin, isolated from Chinese herbs. Cell Mol Immunol. 2013 Nov;10(6):497-505.
- 1,3-Dicaffeoylquinic acid
Catalog No.:BCN2972
CAS No.:19870-46-3
- MLCK inhibitor peptide
Catalog No.:BCC5852
CAS No.:198694-74-5
- Cabralealactone
Catalog No.:BCN4870
CAS No.:19865-87-3
- Alisol B acetate
Catalog No.:BCN2304
CAS No.:19865-76-0
- Alisol A 23-acetate
Catalog No.:BCN3457
CAS No.:19865-75-9
- Tranylcypromine hydrochloride
Catalog No.:BCC7791
CAS No.:1986-47-6
- Fmoc-Ser(tBu)-ol
Catalog No.:BCC2578
CAS No.:198561-87-4
- Fmoc-HoTyr-OH.DCHA
Catalog No.:BCC3246
CAS No.:198560-10-0
- Fmoc-Asparaginol(Trt)
Catalog No.:BCC3042
CAS No.:198543-08-7
- 9,10-Anthracenedione
Catalog No.:BCN3469
CAS No.:19852-76-7
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- Bavachin
Catalog No.:BCN4872
CAS No.:19879-32-4
- Windorphen
Catalog No.:BCC6486
CAS No.:19881-70-0
- Merimepodib
Catalog No.:BCC4128
CAS No.:198821-22-6
- (7R)-Methoxy-8-epi-matairesinol
Catalog No.:BCN7582
CAS No.:198827-23-5
- Z-D-Tyr(tBu)-OH.DCHA
Catalog No.:BCC2744
CAS No.:198828-72-7
- H-D-Phg-OMe.HCl
Catalog No.:BCC3314
CAS No.:19883-41-1
- H-Phe(2-F)-OH
Catalog No.:BCC3222
CAS No.:19883-78-4
- Alisol A
Catalog No.:BCN3455
CAS No.:19885-10-0
- Humulene epoxide II
Catalog No.:BCN4873
CAS No.:19888-34-7
- Continentalic acid
Catalog No.:BCN6526
CAS No.:19889-23-7
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Nagilactone B
Catalog No.:BCN4049
CAS No.:19891-51-1
Bavachinin, as a novel natural pan-PPAR agonist, exhibits unique synergistic effects with synthetic PPAR-gamma and PPAR-alpha agonists on carbohydrate and lipid metabolism in db/db and diet-induced obese mice.[Pubmed:26983922]
Diabetologia. 2016 Jun;59(6):1276-86.
AIMS/HYPOTHESIS: Pan-peroxisome proliferator-activated receptor (PPAR) agonists have long been sought as therapeutics against the metabolic syndrome, but current PPAR agonists show limited efficacy and adverse effects. Natural herbs provide a structurally untapped resource to prevent and treat complicated metabolic syndrome. METHODS: Natural PPAR agonists were screened using reporter gene, competitive binding and 3T3-L1 pre-adipocyte differentiation assays in vitro. The effects on metabolic phenotypes were verified in db/db and diet-induced obese mice. In addition, potentially synergistic actions of Bavachinin (BVC, a novel natural pan-PPAR agonist from the fruit of the traditional Chinese glucose-lowering herb malaytea scurfpea) and synthetic PPAR agonists were studied through nuclear magnetic resonance, molecular docking, reporter gene assays and mouse studies. RESULTS: BVC exhibited glucose-lowering properties without inducing weight gain and hepatotoxicity. Importantly, BVC synergised with thiazolidinediones, which are synthetic PPAR-gamma agonists, and fibrates, which are PPAR-alpha agonists, to induce PPAR transcriptional activity, as well as to lower glucose and triacylglycerol levels in db/db mice. We further found that BVC occupies a novel alternative binding site in addition to the canonical site of synthetic agonists of PPAR, and that the synthetic PPAR-gamma agonist rosiglitazone can block BVC binding to this canonical site but not to the alternative site. CONCLUSIONS/INTERPRETATION: This is the first report of a synergistic glucose- and lipid-lowering effect of BVC and synthetic agonists induced by unique binding with PPAR-gamma or -alpha. This combination may improve the efficacy and decrease the toxicity of marketed drugs for use as adjunctive therapy to treat the metabolic syndrome.
Anti-angiogenic and anti-tumor activity of Bavachinin by targeting hypoxia-inducible factor-1alpha.[Pubmed:22760073]
Eur J Pharmacol. 2012 Sep 15;691(1-3):28-37.
Hypoxia-inducible factor-1 (HIF-1) consists of two subunits, the HIF-1beta, which is constitutively expressed, and HIF-1alpha, which is oxygen-responsive. HIF-1alpha is over-expressed in response to hypoxia, increasing transcriptional activity linked to tumor progression, angiogenesis, metastasis, and invasion. This study aimed to demonstrate that the natural compound, Bavachinin, has potent anti-angiogenic activity in vitro and in vivo. Bavachinin inhibited increases in HIF-1alpha activity in human KB carcinoma (HeLa cell derivative) and human HOS osteosarcoma cells under hypoxia in a concentration-dependent manner, probably by enhancing the interaction between von Hippel-Lindau (VHL) and HIF-1alpha. Furthermore, Bavachinin decreased transcription of genes associated with angiogenesis and energy metabolism that are regulated by HIF-1, such as vascular endothelial growth factors (VEGF), Glut 1 and Hexokinase 2. Bavachinin also inhibited tube formation in human umbilical vein endothelial cells (HUVECs) as well as in vitro migration of KB cells. In vivo studies showed that injecting Bavachinin thrice weekly for four weeks significantly reduced tumor volume and CD31 expression in nude mice with KB xenografts. These data indicate that Bavachinin could be used as a therapeutic agent for inhibiting tumor angiogenesis.
Effect of Bavachinin and its derivatives on T cell differentiation.[Pubmed:24508059]
Int Immunopharmacol. 2014 Apr;19(2):399-404.
Bavachinin, which can be isolated from the Chinese herb Fructus Psoraleae, has the potential as a potent anti-asthma drug. However, the extremely low water solubility of Bavachinin limits its application. In this study, two new derivatives of Bavachinin, i.e., compounds A and B, whose water solubility is better than that of Bavachinin, were synthesized via biotransformation. A comparative investigation was then performed on the effects of these two new derivatives, along with Bavachinin, on T cell differentiation. The results showed that they have different effects. Bavachinin and compound B inhibited green fluorescent protein (GFP) production from the T cells of IL-4-GFP-enhanced transcript (4GET) mice, whereas compound A did not. The effect was mainly attributed to the inhibition of GATA-3 protein production. Bavachinin and compound B can inhibit the production of GATA-3 mRNA, but they showed different effects on the production of T-bet mRNA. Compound B increased the production of T-bet mRNA, whereas Bavachinin did not. The results will be very useful for optimizing Bavachinin so that potent anti-allergic drugs can be developed. The structure-activity relationship of Th2 was revealed based on the difference between Bavachinin and compound B. This finding can enrich the database of preliminary drug screening from their chemical structures.
Separation and peroxisome proliferator-activated receptor-gamma agonist activity evaluation of synthetic racemic bavachinin enantiomers.[Pubmed:25978962]
Bioorg Med Chem Lett. 2015 Jun 15;25(12):2579-83.
Bavachinin, isolated from Psoralea corylifolia seeds, has been reported to demonstrate peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonist activity. However, isolated Bavachinin is actually a mixture of S and R configurations, with an enantiomeric excess value of approximately 24.3%. For further study on the structure-activity relationships of Bavachinin, investigating the PPAR-gamma agonist activity of the two enantiomers is crucial. Considering the limited availability, racemic Bavachinin was prepared in this study using chemical synthesis. The enantiomers of racemic Bavachinin were then separated using supercritical fluid chromatography. This concise strategy yielded (S)- and (R)-Bavachinin in optical purity as high as 97.5%. The PPAR-gamma agonist activity of the two enantiomers was evaluated using a time-resolved fluorescence resonance energy transfer-based competitive binding assay method; IC50 values of (S)- and (R)-Bavachinin were 616.7 and 471.2 nM, respectively. The interaction between the compounds and PPAR-gamma was further explored using a molecular docking method. This study suggests that (S)- and (R)-Bavachinin demonstrate similar PPAR-gamma agonist activities.


