Bazedoxifene acetateEstrogen receptor modulator CAS# 198481-33-3 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- (Z)-2-decenoic acid
Catalog No.:BCC1295
CAS No.:15790-91-7
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Toremifene
Catalog No.:BCC2010
CAS No.:89778-26-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

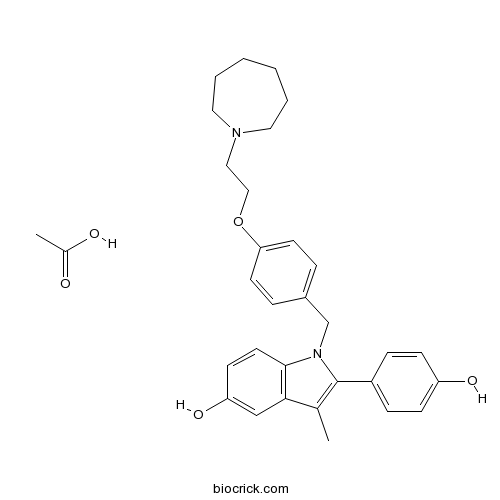
| Cas No. | 198481-33-3 | SDF | Download SDF |
| PubChem ID | 154256 | Appearance | Powder |
| Formula | C32H38N2O5 | M.Wt | 530.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TSE 424; WAY-TES 424 | ||
| Solubility | DMSO : ≥ 100 mg/mL (188.45 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | acetic acid;1-[[4-[2-(azepan-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methylindol-5-ol | ||
| SMILES | CC1=C(N(C2=C1C=C(C=C2)O)CC3=CC=C(C=C3)OCCN4CCCCCC4)C5=CC=C(C=C5)O.CC(=O)O | ||
| Standard InChIKey | OMZAMQFQZMUNTP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H34N2O3.C2H4O2/c1-22-28-20-26(34)12-15-29(28)32(30(22)24-8-10-25(33)11-9-24)21-23-6-13-27(14-7-23)35-19-18-31-16-4-2-3-5-17-31;1-2(3)4/h6-15,20,33-34H,2-5,16-19,21H2,1H3;1H3,(H,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent selective estrogen receptor modulator (SERM) (IC50 values are 26 and 99 nM for ERα and ERβ respectively). Inhibits 17β-estradiol-induced proliferation in MCF-7 cells. Co-treatment with Raloxifene completely abolishes raloxifene-induced stimulation of luminal epithelial cells and myometrium. |

Bazedoxifene acetate Dilution Calculator

Bazedoxifene acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8845 mL | 9.4224 mL | 18.8448 mL | 37.6896 mL | 47.112 mL |
| 5 mM | 0.3769 mL | 1.8845 mL | 3.769 mL | 7.5379 mL | 9.4224 mL |
| 10 mM | 0.1884 mL | 0.9422 mL | 1.8845 mL | 3.769 mL | 4.7112 mL |
| 50 mM | 0.0377 mL | 0.1884 mL | 0.3769 mL | 0.7538 mL | 0.9422 mL |
| 100 mM | 0.0188 mL | 0.0942 mL | 0.1884 mL | 0.3769 mL | 0.4711 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bazedoxifene, a novel selective estrogen receptor modulator (SERM), has been developed to have favorable effects on bone and the lipid profile while minimizing stimulation of uterine or breast tissues. Two large Phase III clinical trials showed that Bazedoxifene, as well as raloxifene, increased bone mineral density, decreased levels of bone turnover markers, and significantly reduced the risk of new vertebral fractures in postmenopausal women compared with placebo.
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- Boc-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC2623
CAS No.:198474-61-2
- Parecoxib Sodium
Catalog No.:BCC4248
CAS No.:198470-85-8
- Parecoxib
Catalog No.:BCC4041
CAS No.:198470-84-7
- Boc-D-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC3308
CAS No.:198470-36-9
- LY 367385
Catalog No.:BCC6983
CAS No.:198419-91-9
- Erythrodiol 3-palmitate
Catalog No.:BCN4869
CAS No.:19833-13-7
- Myricetin 3-O-beta-D-glucopyranoside
Catalog No.:BCN8144
CAS No.:19833-12-6
- Medicagol
Catalog No.:BCN8430
CAS No.:1983-72-8
- Gap 27
Catalog No.:BCC1033
CAS No.:198284-64-9
- Triptobenzene K
Catalog No.:BCN8055
CAS No.:198129-88-3
- 9,10-Anthracenedione
Catalog No.:BCN3469
CAS No.:19852-76-7
- Fmoc-Asparaginol(Trt)
Catalog No.:BCC3042
CAS No.:198543-08-7
- Fmoc-HoTyr-OH.DCHA
Catalog No.:BCC3246
CAS No.:198560-10-0
- Fmoc-Ser(tBu)-ol
Catalog No.:BCC2578
CAS No.:198561-87-4
- Tranylcypromine hydrochloride
Catalog No.:BCC7791
CAS No.:1986-47-6
- Alisol A 23-acetate
Catalog No.:BCN3457
CAS No.:19865-75-9
- Alisol B acetate
Catalog No.:BCN2304
CAS No.:19865-76-0
- Cabralealactone
Catalog No.:BCN4870
CAS No.:19865-87-3
- MLCK inhibitor peptide
Catalog No.:BCC5852
CAS No.:198694-74-5
- 1,3-Dicaffeoylquinic acid
Catalog No.:BCN2972
CAS No.:19870-46-3
- Bavachinin
Catalog No.:BCN4871
CAS No.:19879-30-2
- Bavachin
Catalog No.:BCN4872
CAS No.:19879-32-4
Endometrial profile of bazedoxifene acetate alone and in combination with conjugated equine estrogens in a primate model.[Pubmed:23793168]
Menopause. 2013 Jul;20(7):777-84.
OBJECTIVE: Concerns of breast cancer risk in postmenopausal women taking combined estrogen + progestin therapy have generated interest in the use of selective estrogen receptor modulators (SERMs) as potential progestin alternatives. Endometrial proliferation and cancer risk are major concerns, however, for estrogens and certain types of SERMs when given alone. The primary aim of this study was to evaluate the endometrial profile of Bazedoxifene acetate (BZA), a third-generation SERM, alone and in combination with conjugated equine estrogens (CEE) in a postmenopausal primate model. METHODS: Ninety-eight ovariectomized cynomolgus monkeys (Macaca fascicularis) were randomized to receive no hormone treatment (controls), BZA 20 mg, CEE 0.45 mg, or the combination of BZA 20 mg + CEE 0.45 mg once daily for 20 months in a parallel-arm study design. The primary outcome measure was endometrial epithelial proliferation. RESULTS: BZA + CEE and BZA treatment resulted in significantly less endometrial epithelial area and Ki67 expression compared with CEE (P < 0.001 for all). The prevalence of endometrial hyperplasia and other estrogen-induced morphologic changes in the BZA + CEE and BZA groups was not significantly different from controls. The addition of BZA to CEE completely inhibited the expression of estrogen receptor-alpha-regulated genes (TFF1 and PGR), whereas BZA alone had no effect. BZA + CEE and BZA treatment also resulted in lower estrogen receptor-alpha protein expression in the endometrium compared with the control and CEE groups (P < 0.05 for all). CONCLUSIONS: BZA given at a clinically relevant dose inhibits estrogen effects on the endometrium and lacks uterotropic effects when given alone.
Effects of bazedoxifene acetate with and without conjugated equine estrogens on the breast of postmenopausal monkeys.[Pubmed:23103754]
Menopause. 2012 Nov;19(11):1242-52.
OBJECTIVE: Concerns about increased breast cancer risk with estrogen and progestin therapy have led to an increased interest in progestin alternatives. The main objective of this study was to determine if Bazedoxifene acetate (BZA), a new selective estrogen receptor modulator, will antagonize the proliferative and transcriptional effects of conjugated equine estrogens (CEE) in the breast. METHODS: As part of a 20-month preclinical trial, 95 ovariectomized cynomolgus macaques (Macaca fascicularis) were randomized to receive no treatment or treatment with BZA (20 mg/d), CEE (0.45 mg/d), or BZA and CEE in combination (women's daily equivalent doses). The data presented here include breast effects after 6 months of treatment. Endpoints included histomorphometry, histopathological evaluations, gene microarray assays, polymerase chain reaction quantification of specific estrogen receptor alpha (ER-alpha) activity markers, and immunohistochemical detection of sex steroid receptors, and the proliferation marker Ki67. RESULTS: BZA + CEE and BZA resulted in significantly less total epithelial density, lobular enlargement, and Ki67 immunolabeling in the terminal ducts compared with CEE alone (P < 0.05 for all). The addition of BZA to CEE antagonized the expression of ER-alpha-regulated genes such as GREB1 and TFF1 (P < 0.01 for both), whereas BZA alone had minimal effects on ER-alpha-mediated transcriptional activity. BZA and BZA + CEE did not significantly up-regulate genes related to cell cycle progression and proliferation. BZA with and without CEE also resulted in less lobular and terminal duct ER-alpha immunolabeling compared with control and CEE (P < 0.0001 for all). CONCLUSIONS: These findings demonstrate that BZA given at a clinically relevant dose is an estrogen antagonist in the breast, supporting the idea that CEE + BZA may provide a lower breast cancer risk profile compared with traditional estrogen + progestin therapies.
Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity.[Pubmed:15961563]
Endocrinology. 2005 Sep;146(9):3999-4008.
We assessed the preclinical characteristics of a novel, stringently screened selective estrogen receptor modulator, Bazedoxifene acetate, including its ability to bind to and activate estrogen receptors and promote increased bone mineral density and bone strength in rats, and the effects impacting the uterine endometrium, breast cancer cell proliferation, and central nervous system-associated vasomotor responses in an animal model. Bazedoxifene bound to estrogen receptor-alpha with an IC50 of 26 nm, an affinity similar to that of raloxifene. Bazedoxifene did not stimulate proliferation of MCF-7 cells but did inhibit 17beta-estradiol-induced proliferation with an IC50 of 0.19 nm. In an immature rat uterine model, bazedoxifene (0.5 and 5.0 mg/kg) was associated with less increase in uterine wet weight than either ethinyl estradiol (10 microg/kg) or raloxifene (0.5 and 5.0 mg/kg). Histological analysis revealed that coadministration of bazedoxifene also appeared to reduce raloxifene-stimulated endometrial luminal epithelial cell and myometrial cell hypertrophy. In ovariectomized rats, bazedoxifene was associated with significant increases in bone mineral density at 6 wk, compared with control, and better compressive strength of bone samples from the L4 vertebrae, compared with samples from ovariectomized animals. In the morphine-addicted rat model of vasomotor activity, bone-sparing doses of bazedoxifene alone were not associated with 17beta-estradiol inhibition of increased vasomotor activity. Bazedoxifene acetate represents a promising new treatment for osteoporosis, with a potential for less uterine and vasomotor effects than selective estrogen receptor modulators currently used in clinical practice. Controlled clinical trial data will be needed to confirm these effects.


