Parecoxib SodiumCAS# 198470-85-8 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 198470-85-8 | SDF | Download SDF |
| PubChem ID | 15895902 | Appearance | Powder |
| Formula | C19H17N2NaO4S | M.Wt | 392.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SC 69124A | ||
| Solubility | DMSO : ≥ 100 mg/mL (254.84 mM) *"≥" means soluble, but saturation unknown. | ||
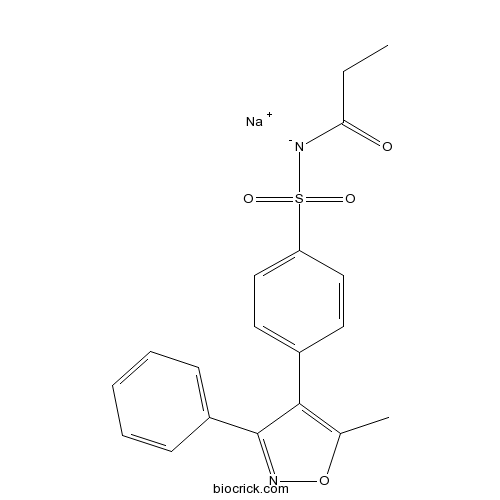
| Chemical Name | sodium;[4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)phenyl]sulfonyl-propanoylazanide | ||
| SMILES | CCC(=O)[N-]S(=O)(=O)C1=CC=C(C=C1)C2=C(ON=C2C3=CC=CC=C3)C.[Na+] | ||
| Standard InChIKey | HQPVVKXJNZEAFW-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C19H18N2O4S.Na/c1-3-17(22)21-26(23,24)16-11-9-14(10-12-16)18-13(2)25-20-19(18)15-7-5-4-6-8-15;/h4-12H,3H2,1-2H3,(H,21,22);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Parecoxib is a potent and selective COX-2 inhibitor.
IC50 value:
Target: COX-2
in vitro: The prodrug Parecoxib as well as its active metabolite val have a specific affinity to the cannabinoid (CB) receptor measured in CB1-expressing HEK 293 cells and rat brain tissue [1].
in vivo: Adult male Sprague-Dawley rats were administered parecoxib (10 or 30 mg kg(-1), IP) or isotonic saline twice a day starting 24 h after middle cerebral artery occlusion (MCAO) for three consecutive days [2]. The selective COX-2 inhibitor parecoxib was delivered 20 min before or 20 min after the incision by intraperitoneal injection. Pretreatment with parecoxib markedly attenuated the pain hypersensitivity induced by incision [3]. References: | |||||

Parecoxib Sodium Dilution Calculator

Parecoxib Sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5484 mL | 12.7421 mL | 25.4842 mL | 50.9684 mL | 63.7105 mL |
| 5 mM | 0.5097 mL | 2.5484 mL | 5.0968 mL | 10.1937 mL | 12.7421 mL |
| 10 mM | 0.2548 mL | 1.2742 mL | 2.5484 mL | 5.0968 mL | 6.371 mL |
| 50 mM | 0.051 mL | 0.2548 mL | 0.5097 mL | 1.0194 mL | 1.2742 mL |
| 100 mM | 0.0255 mL | 0.1274 mL | 0.2548 mL | 0.5097 mL | 0.6371 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Parecoxib is a potent and selective COX-2 inhibitor.
- Parecoxib
Catalog No.:BCC4041
CAS No.:198470-84-7
- Boc-D-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC3308
CAS No.:198470-36-9
- LY 367385
Catalog No.:BCC6983
CAS No.:198419-91-9
- Erythrodiol 3-palmitate
Catalog No.:BCN4869
CAS No.:19833-13-7
- Myricetin 3-O-beta-D-glucopyranoside
Catalog No.:BCN8144
CAS No.:19833-12-6
- Medicagol
Catalog No.:BCN8430
CAS No.:1983-72-8
- Gap 27
Catalog No.:BCC1033
CAS No.:198284-64-9
- Triptobenzene K
Catalog No.:BCN8055
CAS No.:198129-88-3
- GW311616
Catalog No.:BCC5393
CAS No.:198062-54-3
- AM 404
Catalog No.:BCC6945
CAS No.:198022-70-7
- GW311616 hydrochloride
Catalog No.:BCC5394
CAS No.:197890-44-1
- Stachartin E
Catalog No.:BCN6970
CAS No.:1978388-58-7
- Boc-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC2623
CAS No.:198474-61-2
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- 9,10-Anthracenedione
Catalog No.:BCN3469
CAS No.:19852-76-7
- Fmoc-Asparaginol(Trt)
Catalog No.:BCC3042
CAS No.:198543-08-7
- Fmoc-HoTyr-OH.DCHA
Catalog No.:BCC3246
CAS No.:198560-10-0
- Fmoc-Ser(tBu)-ol
Catalog No.:BCC2578
CAS No.:198561-87-4
- Tranylcypromine hydrochloride
Catalog No.:BCC7791
CAS No.:1986-47-6
- Alisol A 23-acetate
Catalog No.:BCN3457
CAS No.:19865-75-9
- Alisol B acetate
Catalog No.:BCN2304
CAS No.:19865-76-0
- Cabralealactone
Catalog No.:BCN4870
CAS No.:19865-87-3
Simultaneous determination of parecoxib sodium and its active metabolite valdecoxib in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study after intravenous and intramuscular administration.[Pubmed:27107851]
J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Jun 1;1022:220-229.
In this study, we developed and validated a new, rapid, specific and sensitive ultra-performance liquid chromatography-tandem mass spectrometric (UPLC-MS/MS) method to simultaneously determine Parecoxib Sodium (PX) and its active metabolite, valdecoxib (VX), in rat plasma. Plasma samples were prepared by plasma protein precipitation combined with a liquid-liquid extraction method. The separation was carried out on a Kinetex C18 column (2.1mmx50mm, 2.6mum) with a gradient elution using methanol (A) and a 2mM ammonium acetate aqueous solution (B). The analysis was performed in less than 3min with a flow rate of 0.2mL/min. Ketoprofen was used as an internal standard (IS). Mass spectrometric detection was conducted with a triple quadrupole detector equipped with electrospray ionization in the negative ion mode (ESI(-)) using multiple reaction monitoring (MRM). The calibration curves were linear over the concentration ranges of 5-4000ng/mL for PX and 5-2000ng/mL for VX with all correlation coefficients greater than 0.998. The intra- and inter-day relative standard deviations (RSD) for both analytes were within 15% and the accuracy was within 85-115% at all quality control levels. The mean extraction recoveries for all analytes obtained from three concentrations of QC plasma samples were more than 89.0% efficient. Selectivity, matrix effect, dilution integrity and stability were also validated. The method was successfully used to investigate the pharmacokinetics of PX and VX in rat plasma after intravenous and intramuscular administration of PX.
[Synergistic analgesic effect of choline and parecoxib sodium in mice and the mechanism].[Pubmed:27881346]
Nan Fang Yi Ke Da Xue Xue Bao. 2016 Nov 20;36(11):1536-1540.
OBJECTIVE: To investigate the synergistic analgesic effect of choline and Parecoxib Sodium and study its mechanism. METHODS: In male Kunming mice with acetic acid-induced writhing, the ED50 of choline and Parecoxib Sodium (administered via the tail vein at 2 h and 30 min before modeling, respectively) and their combined use were determined. In saline (control) group, ED50 choline (C) group, ED50 Parecoxib Sodium (P) group, and 1/2ED50 choline and Parecoxib Sodium (1/2[C+P]) group, blood samples were collected from the eyeball 10 min after intraperitoneal administration of acetic acid to detect the levels of IL-1, TNF-alpha, PGE2, NF-kappaB, and I-kappaB levels using ELISA kits. RESULTS: In the acetic acid-induced writhing model, the ED50 of choline and Parecoxib Sodium was 8.64 and 6.33 mg/kg, and when combined, their ED50 was 2.13 and 1.56 mg/kg, respectively. The isobolograms of Parecoxib Sodium and choline showed that the measured ED50 of the two drugs combined was below the theoretical ED50 value (P<0.05) with a combination index (CI) of <0.9. Compared with the control group, C group, P group, and 1/2 (C+P) group all showed significantly lowered IL-1 and TNF-alpha levels (P<0.05), especially in 1/2 (C+P) group (P<0.05). PGE2 level was significantly lower in P group and 1/2 (C+P) group compared with the control group (P<0.05). NF-kappaB and I-kappaB levels were significantly lowered in C, P, and 1/2 (C+P) groups (P<0.05), and the reduction was the most obvious in 1/2 (C+P) group (P<0.05). CONCLUSION: Choline and Parecoxib Sodium has a synergistic analgesic effect, and their interactions may involve the in vivo expression of NF-kappaB.
Parecoxib sodium pretreatment reduces myoclonus after etomidate: A prospective, double-blind, randomized clinical trial.[Pubmed:28291508]
Int J Clin Pharmacol Ther. 2017 Jul;55(7):601-605.
OBJECTIVE: Myoclonus induced by etomidate during induction of general anesthesia is a common phenomenon. This prospective, randomized, saline-controlled clinical study was performed to evaluate the effect of Parecoxib Sodium pretreatment on the incidence and severity of etomidate-induced myoclonus. METHODS: 60 patients, American Society of Anesthesiologists (ASA) physical status I or II, aged 20 to 60 years, who were scheduled to undergo elective laparoscopic cholecystectomy under general anesthesia, were allocated randomly into one of two groups to receive Parecoxib Sodium 40 mg intravenous (group P, n = 30) or the same volume of saline (group S, n = 30) 30 minutes before administration of etomidate (0.3 mg/kg). Myoclonus was assessed on a scale of 0 - 3. Postoperative side effects were recorded. RESULTS: The two groups were comparable with regard to baseline characteristics. The incidence of myoclonus was significantly lower in the Parecoxib Sodium group (11/30; 37%) than in the saline group (21/30; 70%) (p < 0.05). The severity of myoclonic movements was also significantly reduced by Parecoxib Sodium (p < 0.05). There were no significant differences between the two groups with respect to postoperative side effects. CONCLUSIONS: Pretreatment with intravenous injection of Parecoxib Sodium 40 mg significantly reduced the incidence and severity of etomidate-induced myoclonus without significant side effects..
Efficacy and safety of Postoperative Intravenous Parecoxib sodium Followed by ORal CElecoxib (PIPFORCE) post-total knee arthroplasty in patients with osteoarthritis: a study protocol for a multicentre, double-blind, parallel-group trial.[Pubmed:27609846]
BMJ Open. 2016 Sep 8;6(9):e011732.
INTRODUCTION: Total knee arthroplasty (TKA) has been regarded as a most painful orthopaedic surgery. Although many surgeons sequentially use parecoxib and celecoxib as a routine strategy for postoperative pain control after TKA, high quality evidence is still lacking to prove the effect of this sequential regimen, especially at the medium-term follow-up. The purpose of this study, therefore, is to evaluate efficacy and safety of postoperative intravenous Parecoxib Sodium followed by oral celecoxib in patients with osteoarthritis (OA) undergoing TKA. The hypothesis is that compared to placebo with opioids as rescue treatment, sequential use of parecoxib and celecoxib can achieve less morphine consumption over the postoperative 2 weeks, as well as better pain control, quicker functional recovery in the postoperative 6 weeks and less opioid-related adverse events during the 12-week recovery phase. METHODS AND ANALYSIS: This study is designed as a multicentre, randomised, double-blind, parallel-group and placebo-controlled trial. The target sample size is 246. All participants who meet the study inclusion and exclusion criteria will be randomly assigned in a 1:1 ratio to either the parecoxib/celecoxib group or placebo group. The randomisation and allocation will be study site based. The study will consist of three phases: an initial screening phase; a 6-week double-blind treatment phase; and a 6-week follow-up phase. The primary end point is cumulative opioid consumption during 2 weeks postoperation. Secondary end points consist of the postoperative visual analogue scale score, knee joint function, quality of life, local skin temperature, erythrocyte sedimentation rate, C reactive protein, cytokines and blood coagulation parameters. Safety end points will be monitored too. ETHICS AND DISSEMINATION: Ethics approval for this study has been obtained from the Ethics Committee, Peking Union Medical College Hospital, China (Protocol number: S-572) Study results will be available as published manuscripts and presentations at national and international meetings. TRIAL REGISTRATION NUMBER: NCT02198924.


