Bakkenolide ACAS# 19906-72-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19906-72-0 | SDF | Download SDF |
| PubChem ID | 442173 | Appearance | Cryst. |
| Formula | C15H22O2 | M.Wt | 234.34 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
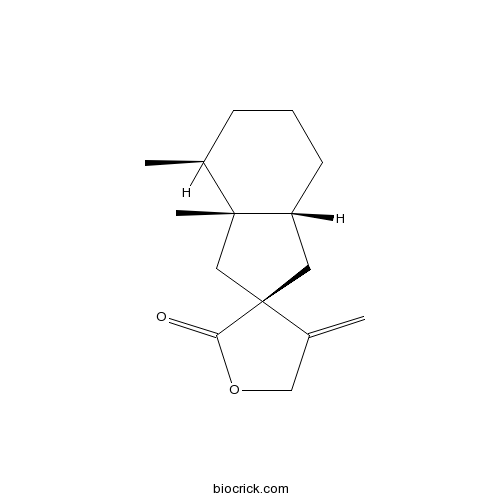
| Chemical Name | (2R,3aR,7S,7aR)-7,7a-dimethyl-4'-methylidenespiro[3,3a,4,5,6,7-hexahydro-1H-indene-2,3'-oxolane]-2'-one | ||
| SMILES | CC1CCCC2C1(CC3(C2)C(=C)COC3=O)C | ||
| Standard InChIKey | OVXAYHNZXBOVPV-QMGNLALYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Bakkenolide A has antifeedant and growth inhibitory effects on neonate variegated cutworms. 2. Bakkenolide A inhibits leukemia by regulation of HDAC3 and PI3K/Akt-related signaling pathways. |
| Targets | HDAC | IkB | IL Receptor | Caspase | TNF-α | PI3K | Akt | GSK-3 | IKK |

Bakkenolide A Dilution Calculator

Bakkenolide A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2673 mL | 21.3365 mL | 42.673 mL | 85.3461 mL | 106.6826 mL |
| 5 mM | 0.8535 mL | 4.2673 mL | 8.5346 mL | 17.0692 mL | 21.3365 mL |
| 10 mM | 0.4267 mL | 2.1337 mL | 4.2673 mL | 8.5346 mL | 10.6683 mL |
| 50 mM | 0.0853 mL | 0.4267 mL | 0.8535 mL | 1.7069 mL | 2.1337 mL |
| 100 mM | 0.0427 mL | 0.2134 mL | 0.4267 mL | 0.8535 mL | 1.0668 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydromethysticin
Catalog No.:BCN2476
CAS No.:19902-91-1
- Syringaresinol diacetate
Catalog No.:BCN4874
CAS No.:1990-77-8
- Kaempferol 3-O-beta-sophoroside
Catalog No.:BCN3336
CAS No.:19895-95-5
- 29-Nor-20-oxolupeol
Catalog No.:BCN6678
CAS No.:19891-85-1
- Jaborosalactone D
Catalog No.:BCN7946
CAS No.:19891-82-8
- Nagilactone B
Catalog No.:BCN4049
CAS No.:19891-51-1
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Continentalic acid
Catalog No.:BCN6526
CAS No.:19889-23-7
- Humulene epoxide II
Catalog No.:BCN4873
CAS No.:19888-34-7
- Alisol A
Catalog No.:BCN3455
CAS No.:19885-10-0
- H-Phe(2-F)-OH
Catalog No.:BCC3222
CAS No.:19883-78-4
- H-D-Phg-OMe.HCl
Catalog No.:BCC3314
CAS No.:19883-41-1
- Maackiain
Catalog No.:BCN1236
CAS No.:19908-48-6
- H-Phe(4-Me)-OH
Catalog No.:BCC3270
CAS No.:1991-87-3
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- T 98475
Catalog No.:BCC7395
CAS No.:199119-18-1
- Furanodiene
Catalog No.:BCN6454
CAS No.:19912-61-9
- Chamigrenal
Catalog No.:BCN7847
CAS No.:19912-84-6
- Futoenone
Catalog No.:BCN6408
CAS No.:19913-01-0
- Enniatin B1
Catalog No.:BCN4853
CAS No.:19914-20-6
- Boc-D-N-Me-Ala-OH
Catalog No.:BCC3211
CAS No.:19914-38-6
- O6-Benzylguanine
Catalog No.:BCC6485
CAS No.:19916-73-5
- Dehydroglyasperin C
Catalog No.:BCN6790
CAS No.:199331-35-6
- Glyurallin A
Catalog No.:BCN7538
CAS No.:199331-36-7
Bakkenolide A inhibits leukemia by regulation of HDAC3 and PI3K/Akt-related signaling pathways.[Pubmed:27522258]
Biomed Pharmacother. 2016 Oct;83:958-966.
Leukemia has been the third type of cancer killing many people across the world. Bakkenolide A (Bak), extracted from Petasites tricholobus, has been suggested to against cancer and display protective effects on inflammatory cytokines formation. And increasing evidences suggest that histone deacetylase 3 (HDAC3) plays vital roles in cancer formation and persistence via cell death, apoptosis and inflammation. But the function of Bakkenolide A in regulating leukemia is not understood yet, particularly via HDAC3. Here, we found that HDAC3 is up-regulated in clinical samples of leukemia compared with adjacent normal tissues. Then the expression of HDAC3 was knocked down via RNA interference in K562 cells. And inhibition of HDAC3 expression is able to improve leukemia invasion, migration and proliferation. Further, we also found HDAC3 bound to IkappaBalpha, affecting subsequent inflammation response. Moreover, Bakkenolide A was found to inhibit inflammation, induce apoptosis and cell death in leukemia cells via PI3K-regulated signaling pathway, down-regulating IKKs expression and suppressing in proinflammatory cytokines of IL-1beta, IL-18 and TNF-alpha. Up-regulation of Caspase3/7 was observed in cells of HDAC3-knockdown and Bakkenolide A treatment, inducing leukemia cell apoptosis. Also, the expression of Akt and GSK were activated by HDAC3-knockdown and Bakkenolide A-treatment. Thus, these results indicated that Bakkenolide A-mediated HDAC3 sensitization in leukemia cells seem to be associated with activation of effector IKKs, Akt/GSK, and caspases through induction of the PI3K pathway, leading to inflammation, cell death, and apoptosis.
Determination of bakkenolide A in rat plasma using liquid chromatography/tandem mass spectrometry and its application to a pharmacokinetic study.[Pubmed:22899504]
J Mass Spectrom. 2012 Aug;47(8):962-8.
A sensitive rapid analytical method was established and validated to determine the Bakkenolide A (BA) in rat plasma. This method was further applied to assess the pharmacokinetics of BA in rats receiving a single dose of BA. Liquid chromatography tandem mass spectrometry in multiple reaction monitoring mode was used in the method, and costundide was used as internal standard. A simple protein precipitation based on methanol was employed. The combination of a simple sample cleanup and short chromatographic running time (2.4 min) increased the throughput of the method substantially. The method was validated over the range of 1-1000 ng/mL with a correlation coefficient > 0.99. The lower limit of quantification was 1 ng/mL for BA in plasma. Intra- and inter-day accuracies for BA were 93-112% and 103-104%, respectively, and the inter-day precision was less than 15%. After a single oral dose of 20 mg/kg of BA, the mean peak plasma concentration (C(max) ) of BA was 234.7 +/- 161 ng/mL at 0.25 h. The area under the plasma concentration-time curve (AUC(0-24 h) ) was 535.8 +/- 223.7 h.ng/mL, and the elimination half-life (T(1/2) ) was 5.0 +/- 0.36 h. In case of intravenous administration of BA at a dosage of 2 mg/kg, the area under the plasma concentration-time curve (AUC(0-24 h) ) was 342 +/- 98 hng/mL, and the elimination half-life (T(1/2) ) was 5.8 +/- 0.7 h. Based on the results, the oral bioavailability of BA in rats at 20 mg/kg is 15.7%.


