FutoenoneCAS# 19913-01-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

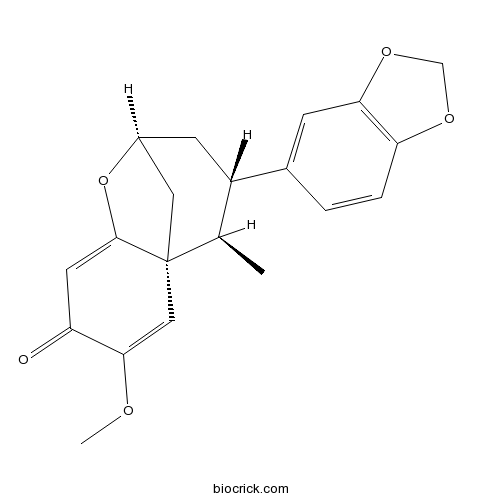
| Cas No. | 19913-01-0 | SDF | Download SDF |
| PubChem ID | 9819306 | Appearance | Powder |
| Formula | C20H20O5 | M.Wt | 340.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1C(CC2CC13C=C(C(=O)C=C3O2)OC)C4=CC5=C(C=C4)OCO5 | ||
| Standard InChIKey | SXHVHWXETMBKPP-KXXATPMCSA-N | ||
| Standard InChI | InChI=1S/C20H20O5/c1-11-14(12-3-4-16-17(5-12)24-10-23-16)6-13-8-20(11)9-18(22-2)15(21)7-19(20)25-13/h3-5,7,9,11,13-14H,6,8,10H2,1-2H3/t11-,13+,14+,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Futoenone and a series of its derivatives have shown inhibitory activities against matrix metalloproteinases, the molecular modelings of these compounds indicate the preferred binding of the P2′ site of the enzymes. 2. Neolignan derivative compounds of the 2,4-diaryl-1,3-dithiolane and futoenone variety exhibit both platelet activating factor receptor(PAF)and 5-lipoxygenase antagonist activity. |
| Targets | PAFR |

Futoenone Dilution Calculator

Futoenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9377 mL | 14.6886 mL | 29.3772 mL | 58.7544 mL | 73.443 mL |
| 5 mM | 0.5875 mL | 2.9377 mL | 5.8754 mL | 11.7509 mL | 14.6886 mL |
| 10 mM | 0.2938 mL | 1.4689 mL | 2.9377 mL | 5.8754 mL | 7.3443 mL |
| 50 mM | 0.0588 mL | 0.2938 mL | 0.5875 mL | 1.1751 mL | 1.4689 mL |
| 100 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5875 mL | 0.7344 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chamigrenal
Catalog No.:BCN7847
CAS No.:19912-84-6
- Furanodiene
Catalog No.:BCN6454
CAS No.:19912-61-9
- T 98475
Catalog No.:BCC7395
CAS No.:199119-18-1
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- H-Phe(4-Me)-OH
Catalog No.:BCC3270
CAS No.:1991-87-3
- Maackiain
Catalog No.:BCN1236
CAS No.:19908-48-6
- Bakkenolide A
Catalog No.:BCN5402
CAS No.:19906-72-0
- Dihydromethysticin
Catalog No.:BCN2476
CAS No.:19902-91-1
- Syringaresinol diacetate
Catalog No.:BCN4874
CAS No.:1990-77-8
- Kaempferol 3-O-beta-sophoroside
Catalog No.:BCN3336
CAS No.:19895-95-5
- 29-Nor-20-oxolupeol
Catalog No.:BCN6678
CAS No.:19891-85-1
- Jaborosalactone D
Catalog No.:BCN7946
CAS No.:19891-82-8
- Enniatin B1
Catalog No.:BCN4853
CAS No.:19914-20-6
- Boc-D-N-Me-Ala-OH
Catalog No.:BCC3211
CAS No.:19914-38-6
- O6-Benzylguanine
Catalog No.:BCC6485
CAS No.:19916-73-5
- Dehydroglyasperin C
Catalog No.:BCN6790
CAS No.:199331-35-6
- Glyurallin A
Catalog No.:BCN7538
CAS No.:199331-36-7
- Crocatone
Catalog No.:BCN3532
CAS No.:19937-86-1
- Kolavenol
Catalog No.:BCN4680
CAS No.:19941-83-4
- Gymnestrogenin
Catalog No.:BCN7846
CAS No.:19942-02-0
- 3-Epicabraleadiol
Catalog No.:BCN4875
CAS No.:19942-04-2
- Y-33075
Catalog No.:BCC2064
CAS No.:199433-58-4
- Veraguensin
Catalog No.:BCN2163
CAS No.:19950-55-1
- 2-Amino-4-chlorobenzothiazole
Catalog No.:BCC8529
CAS No.:19952-47-7
[Study on the bioactive constituents of Piper wallichii].[Pubmed:22734410]
Zhong Yao Cai. 2012 Jan;35(1):53-6.
OBJECTIVE: To investigate the bioactive constituents in the stem of Piper wallichii. METHODS: Compounds were separated by column chromatography of silica gel, ODS-A and Sephadex LH-20. Their structures were elucidated based on spectral analysis. DPPH scavenging activity and AchE inhibitory activity were tested. RESULTS: 10 compounds were isolated and their structures were identified as 3,4-methylenedioxy-benzoic acid (1), vanillic acid (2), benzoic acid (3), N-p-coumaroyltyramine (4), Futoenone (5), futoquinol (6), isofutoquinol A (7), 4-hydroxy-3,5-dimethoxy-benzoic acid (8), futoamide (9), dihydropiperlonguminine (10). CONCLUSION: Compounds 1-6 are isolated from P. wallichii for the first time. Vanillic acid (2) and 4-hydroxy-3,5-dimethoxy-benzoic acid (8) show scavenging activity against DPPH radical with ED50 at 224.33 microg/mL and 11.44 microg/mL, respectively. No compound shows inhibition activity against AchE.
Isolation and purification of seven lignans from Magnolia sprengeri by high-speed counter-current chromatography.[Pubmed:22080044]
J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Dec 1;879(31):3775-9.
Seven lignans including (-)-maglifloenone, Futoenone, magnoline, cylohexadienone, fargesone C, fargesone A and fargesone B were isolated and purified from Magnolia sprengeri Pamp. using high-speed counter-current chromatography (HSCCC) with two-step separation. In the first step, a stepwise elution mode with the two-phase solvent system composed of petroleum ether-ethyl acetate-methanol-water (1:0.8:0.6:1.2, 1:0.8:0.8:1, v/v) was used and 15.6 mg of (-)-maglifloenone, 19.2 mg of Futoenone, 10.8 mg of magnoline, 14.7 mg of cylohexadienone and 217 mg residues were obtained from 370 mg crude extract. In the second step, the residues were successfully separated by HSCCC with the solvent system composed of petroleum ether-ethyl acetate-methanol-water (1:0.8:1.2:0.6, v/v), yielding 33.2 mg of fargesone C, 47.5 mg of fargesone A and 17.7 mg of fargesone B. The purities of the separated compounds were all over 95% determined by HPLC. The chemical structures of these compounds were confirmed by (1)H NMR, (13)C NMR and ESI-MS.
Chemical and biochemical characterization of lignan analogs as novel PAF receptor antagonists.[Pubmed:1668110]
Lipids. 1991 Dec;26(12):1154-6.
Various derivatives and isosteres of neolignans of the 2,5-diaryl tetrahydrofuran type have been synthesized as antagonists of platelet-activating factor (PAF). A detailed analysis of their structure-activity relationship (SAR) has revealed a clear preference for an asymmetrical molecular configuration with a high degree of stereo and chiral specificity associated with greater potency. The trans-2S,5S enantiomers are generally 10-200 times more potent in vitro than their corresponding cis or trans-2R,5R isomers. A similar stereochemical preference is indicated by the recently reported PAF antagonist MK-287 which has undergone clinical evaluation. An azido derivative L-662,025 has been characterized as a photolabile irreversible antagonist of PAF for the investigation of solubilized and partially purified PAF binding proteins from cell membranes. The biological justification for concomitant inhibition of both PAF receptor and 5-lipoxygenase in inflammation is well recognized. The feasibility of developing such dual-functional agents has been demonstrated by a group of dithiolane analogs of neolignans and several derivatives of Futoenone.


