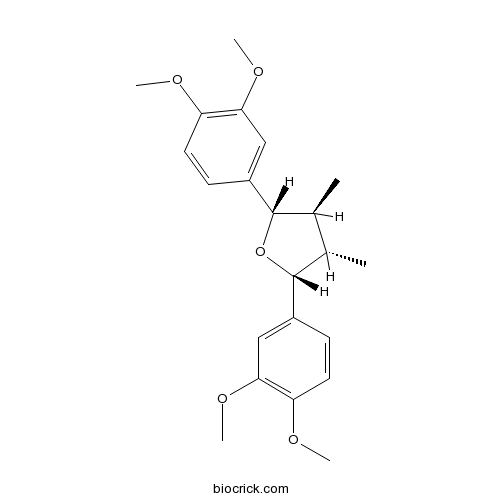
VeraguensinCAS# 19950-55-1 |
- (±)-Galgravin
Catalog No.:BCN8283
CAS No.:528-63-2
- Galbelgin
Catalog No.:BCN9387
CAS No.:10569-12-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19950-55-1 | SDF | Download SDF |
| PubChem ID | 443026 | Appearance | Powder |
| Formula | C22H28O5 | M.Wt | 372.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3S,4S,5S)-2,5-bis(3,4-dimethoxyphenyl)-3,4-dimethyloxolane | ||
| SMILES | CC1C(C(OC1C2=CC(=C(C=C2)OC)OC)C3=CC(=C(C=C3)OC)OC)C | ||
| Standard InChIKey | JLJAVUZBHSLLJL-GKHNXXNSSA-N | ||
| Standard InChI | InChI=1S/C22H28O5/c1-13-14(2)22(16-8-10-18(24-4)20(12-16)26-6)27-21(13)15-7-9-17(23-3)19(11-15)25-5/h7-14,21-22H,1-6H3/t13-,14-,21-,22+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Veraguensin shows activity against trypomastigote T. cruzi. 2. Veraguensin and galgravin can inhibit bone resorption and may offer novel compounds for the development of drugs to treat bone-destructive diseases such as osteoporosis. 3. Veraguensin shows high antileishmanial activity. |
| Targets | NF-kB | p38MAPK | Antifection |

Veraguensin Dilution Calculator

Veraguensin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6846 mL | 13.4228 mL | 26.8456 mL | 53.6913 mL | 67.1141 mL |
| 5 mM | 0.5369 mL | 2.6846 mL | 5.3691 mL | 10.7383 mL | 13.4228 mL |
| 10 mM | 0.2685 mL | 1.3423 mL | 2.6846 mL | 5.3691 mL | 6.7114 mL |
| 50 mM | 0.0537 mL | 0.2685 mL | 0.5369 mL | 1.0738 mL | 1.3423 mL |
| 100 mM | 0.0268 mL | 0.1342 mL | 0.2685 mL | 0.5369 mL | 0.6711 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Y-33075
Catalog No.:BCC2064
CAS No.:199433-58-4
- 3-Epicabraleadiol
Catalog No.:BCN4875
CAS No.:19942-04-2
- Gymnestrogenin
Catalog No.:BCN7846
CAS No.:19942-02-0
- Kolavenol
Catalog No.:BCN4680
CAS No.:19941-83-4
- Crocatone
Catalog No.:BCN3532
CAS No.:19937-86-1
- Glyurallin A
Catalog No.:BCN7538
CAS No.:199331-36-7
- Dehydroglyasperin C
Catalog No.:BCN6790
CAS No.:199331-35-6
- O6-Benzylguanine
Catalog No.:BCC6485
CAS No.:19916-73-5
- Boc-D-N-Me-Ala-OH
Catalog No.:BCC3211
CAS No.:19914-38-6
- Enniatin B1
Catalog No.:BCN4853
CAS No.:19914-20-6
- Futoenone
Catalog No.:BCN6408
CAS No.:19913-01-0
- Chamigrenal
Catalog No.:BCN7847
CAS No.:19912-84-6
- 2-Amino-4-chlorobenzothiazole
Catalog No.:BCC8529
CAS No.:19952-47-7
- NNC 26-9100
Catalog No.:BCC7361
CAS No.:199522-35-5
- Lucidone
Catalog No.:BCN4876
CAS No.:19956-53-7
- Methyllucidone
Catalog No.:BCN4877
CAS No.:19956-54-8
- JIB-04
Catalog No.:BCC4548
CAS No.:199596-05-9
- CP 465022 hydrochloride
Catalog No.:BCC7520
CAS No.:199655-36-2
- Ro 61-8048
Catalog No.:BCC7619
CAS No.:199666-03-0
- LY 334370 hydrochloride
Catalog No.:BCC7559
CAS No.:199673-74-0
- Liguiritigenin-7-O-D-apiosyl-4'-O-D-glucoside
Catalog No.:BCN2840
CAS No.:199796-12-8
- 1-Actamido-3,5-dimethyladmantane
Catalog No.:BCC8449
CAS No.:19982-07-1
- Stattic
Catalog No.:BCC1176
CAS No.:19983-44-9
- PD 166793
Catalog No.:BCC2376
CAS No.:199850-67-4
Design and synthesis of a new series of 3,5-disubstituted isoxazoles active against Trypanosoma cruzi and Leishmania amazonensis.[Pubmed:28152426]
Eur J Med Chem. 2017 Mar 10;128:25-35.
Chagas disease and leishmaniasis are neglected tropical diseases (NTDs) endemic in developing countries. Although there are drugs available for their treatment, efforts on finding new efficacious therapies are continuous. The natural lignans grandisin (1) and Veraguensin (2) show activity against trypomastigote T. cruzi and their scaffold has been used as inspiration to design new derivatives with improved potency and chemical properties. We describe here the planning and microwave-irradiated synthesis of 26 isoxazole derivatives based on the structure of the lignans 1 and 2. In addition, the in vitro evaluation against culture trypomastigotes and intracellular amastigotes of T. cruzi and intracellular amastigotes of L. amazonensis and L. infantum is reported. Among the synthesized derivatives, compounds 17 (IC50 = 5.26 muM for T. cruzi), 29 (IC50 = 1.74 muM for T. cruzi) and 31 (IC50 = 1.13 muM for T. cruzi and IC50 = 5.08 muM for L. amazonensis) were the most active and were also evaluated against recombinant trypanothione reductase of T. cruzi in a preliminary study of their mechanism of action.
In vitro antileishmanial and antimalarial activities of tetrahydrofuran lignans isolated from Nectandra megapotamica (Lauraceae).[Pubmed:18688887]
Phytother Res. 2008 Oct;22(10):1307-10.
Seven tetrahydrofuran lignans, isolated from Nectandra megapotamica (Lauraceae), were evaluated for their in vitro antileishmanial and antimalarial activities. Among the evaluated compounds, machilin-G (1a) and Veraguensin (2a) showed the highest antileishmanial activities, displaying for both compounds an IC(50) value of 18 microg/mL and an IC(90) value of 36 microg/mL, while galgravin (1b), nectandrin-A (1c), nectandrin-B (1d), calopeptin (2b) and ganshisandrine (3) were inactive against Leishmania donovani. In the antimalarial assay against Plasmodium falciparum, it was observed that calopeptin (2b) displayed moderate activity, with IC(50) values of 3800 ng/mL (D6 clone) and 3900 ng/mL (W2 clone), while the lignans 1a-1d, 2a and 3 were inactive. In order to compare the effect on the parasites with toxicity to mammalian cells, the cytotoxic activity of the isolated compounds were evaluated against the Vero cells, showing that all evaluated tetrahydrofuran lignans exhibited no cytotoxicity at the maximum dose tested.
Effects of veraguensin and galgravin on osteoclast differentiation and function.[Pubmed:22526488]
Cytotechnology. 2012 May;64(3):315-22.
The dried flower buds of Magnolia sp. are widely used as herbal medicines because of their anti-inflammatory, anti-malarial and anti-platelet activities. Here, we found that Veraguensin and galgravin, lignan compounds derived from Magnolia sp., dose-dependently inhibited osteoclast formation in co-cultures of bone marrow cells and osteoblastic cells. These compounds also inhibited receptor activator of nuclear factor kappaB ligand (RANKL)-induced osteoclast differentiation in RAW264.7 cells and bone marrow macrophages. In the RANKL-induced signaling pathway, Veraguensin and galgravin reduced p38 phosphorylation and suppressed the expression of c-Fos, a key transcription factor for osteoclastogenesis. Veraguensin and galgravin also inhibited osteoclastic pit formation, which was accompanied by decreased mature osteoclast viability. In conclusion, these results indicate that Veraguensin and galgravin can inhibit bone resorption and may offer novel compounds for the development of drugs to treat bone-destructive diseases such as osteoporosis.


