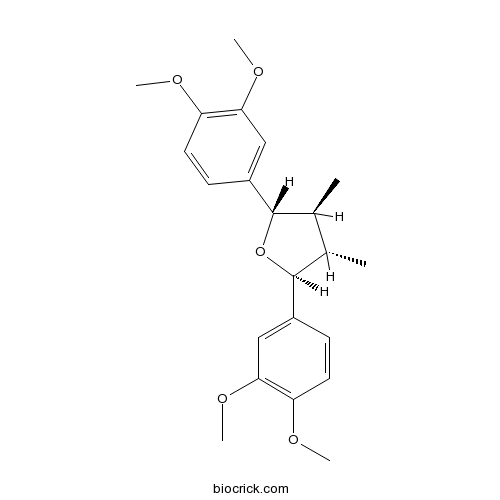
GalbelginCAS# 10569-12-7 |
- Veraguensin
Catalog No.:BCN2163
CAS No.:19950-55-1
- (±)-Galgravin
Catalog No.:BCN8283
CAS No.:528-63-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10569-12-7 | SDF | Download SDF |
| PubChem ID | 11975378 | Appearance | Powder |
| Formula | C22H28O5 | M.Wt | 372.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3S,4S,5S)-2,5-bis(3,4-dimethoxyphenyl)-3,4-dimethyloxolane | ||
| SMILES | CC1C(C(OC1C2=CC(=C(C=C2)OC)OC)C3=CC(=C(C=C3)OC)OC)C | ||
| Standard InChIKey | JLJAVUZBHSLLJL-WJWAULOUSA-N | ||
| Standard InChI | InChI=1S/C22H28O5/c1-13-14(2)22(16-8-10-18(24-4)20(12-16)26-6)27-21(13)15-7-9-17(23-3)19(11-15)25-5/h7-14,21-22H,1-6H3/t13-,14-,21-,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Galbelgin Dilution Calculator

Galbelgin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6846 mL | 13.4228 mL | 26.8456 mL | 53.6913 mL | 67.1141 mL |
| 5 mM | 0.5369 mL | 2.6846 mL | 5.3691 mL | 10.7383 mL | 13.4228 mL |
| 10 mM | 0.2685 mL | 1.3423 mL | 2.6846 mL | 5.3691 mL | 6.7114 mL |
| 50 mM | 0.0537 mL | 0.2685 mL | 0.5369 mL | 1.0738 mL | 1.3423 mL |
| 100 mM | 0.0268 mL | 0.1342 mL | 0.2685 mL | 0.5369 mL | 0.6711 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Garcimultiflorone J
Catalog No.:BCN9386
CAS No.:2010216-41-6
- Petiolin F
Catalog No.:BCN9385
CAS No.:1204251-10-4
- Cynanoside F
Catalog No.:BCN9384
CAS No.:1800029-50-8
- Periplocoside F
Catalog No.:BCN9383
CAS No.:119902-17-9
- Heynic acid
Catalog No.:BCN9382
CAS No.:88478-14-2
- Cucurbitacin V
Catalog No.:BCN9381
CAS No.:152340-37-9
- Thalibealine
Catalog No.:BCN9380
CAS No.:351531-39-0
- Pistaciamide
Catalog No.:BCN9379
CAS No.:1029004-83-8
- 7α-Methoxy-5α,6α-epoxyergosta-8(14),22-dien-3β-ol
Catalog No.:BCN9378
CAS No.:1207441-49-3
- 1,7-Bis(4-hydroxyphenyl)-5-hydroxyhept-1-en-3-one
Catalog No.:BCN9377
CAS No.:1426059-89-3
- (6R,9R)-3-Oxo-α-ionol glucoside
Catalog No.:BCN9376
CAS No.:77699-19-5
- Juncusol 7-O-glucoside
Catalog No.:BCN9375
CAS No.:175094-15-2
- Sericic acid
Catalog No.:BCN9388
CAS No.:55306-03-1
- 16-Desoxycucurbitacin V
Catalog No.:BCN9389
CAS No.:2081098-85-1
- Magnosalin
Catalog No.:BCN9390
CAS No.:81861-74-7
- Isorhapontin
Catalog No.:BCN9391
CAS No.:32727-29-0
- Macarangioside D
Catalog No.:BCN9392
CAS No.:819870-23-0
- 3-O-(6'-O-Galloyl)-β-D-glucopyranosylmaltol
Catalog No.:BCN9393
CAS No.:163397-38-4
- [12]-Dehydrogingerdione
Catalog No.:BCN9394
CAS No.:99742-05-9
- Guttiferone G
Catalog No.:BCN9395
CAS No.:666174-75-0
- Methoxyadiantifoline
Catalog No.:BCN9396
CAS No.:115452-09-0
- Apocynoside I
Catalog No.:BCN9397
CAS No.:358721-31-0
- 10-Dehydrogingerdione
Catalog No.:BCN9398
CAS No.:99742-04-8
- Batatasin I
Catalog No.:BCN9399
CAS No.:51415-00-0
Rapid purification of diastereoisomers from Piper kadsura using supercritical fluid chromatography with chiral stationary phases.[Pubmed:28641835]
J Chromatogr A. 2017 Aug 4;1509:141-146.
Supercritical fluid chromatography (SFC) with chiral stationary phases (CSPs) is an advanced solution for the separation of achiral compounds in Piper kadsura. Analogues and stereoisomers are abundant in natural products, but there are obstacles in separation using conventional method. In this paper, four lignan diastereoisomers, (-)-Galbelgin, (-)-Ganschisandrin, Galgravin and (-)-Veraguensin, from Piper kadsura were separated and purified by chiral SFC. Purification strategy was designed, considering of the compound enrichment, sample purity and purification throughput. Two-step achiral purification method on chiral preparative columns with stacked automated injections was developed. Unconventional mobile phase modifier dichloromethane (DCM) was applied to improve the sample solubility. Four diastereoisomers was prepared at the respective weight of 103.1mg, 10.0mg, 152.3mg and 178.6mg from 710mg extract with the purity of greater than 98%.
Asymmetric synthesis of (+)-galbelgin, (-)-kadangustin J, (-)-cyclogalgravin and (-)-pycnanthulignenes A and B, three structurally distinct lignan classes, using a common chiral precursor.[Pubmed:21749139]
J Org Chem. 2011 Aug 19;76(16):6636-48.
The enantioselective synthesis of three structurally distinct classes of lignan from a single, aza-Claisen-derived, chiral morpholine amide is reported. The class of lignan formed is dependent on the substitution pattern in the aryl rings and choice of protecting group on a key benzylic hydroxyl group. The methodology has been used to asymmetrically synthesize and determine the absolute stereochemistry of lignans (+)-cyclogalgravin 3, (-)-pycnanthulignene A 4, (-)-pycnanthulignene B 5, and (-)-kadangustin J 8.
[Neolignans and lignan from Piper wallichii].[Pubmed:20394289]
Zhongguo Zhong Yao Za Zhi. 2010 Jan;35(2):180-2.
To investigate the chemical constituents of the aerial part of Piper wallichii. Nine compounds were isolated by various chromatographic techniques and the structures were elucidated by their physicochemical properties and the spectral data analysis. Nine compounds were identified as one lignan (-)-Galbelgin (1) and eight neolignans: denudatin B (2), hancinone D (3), (+)-licarin A (4), kadsurenone (5), wallichinine (6), hancinone C (7), hancinone B (8), (+)-burchellin (9). Compounds 1, 3, 4, 8, 9 were isolated from this plant for the first time.
Compounds with neuroprotective activity from the medicinal plant Machilus thunbergii.[Pubmed:19555186]
J Enzyme Inhib Med Chem. 2009 Oct;24(5):1117-21.
The dichloromethane fraction of the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae) significantly protected primary cultures of rat cortical cells exposed to the excitotoxic amino acid, L-glutamate. Through the activity-guided isolation from the CH(2)Cl(2) fraction, (+)-9'-hydroxyGalbelgin (1), isogalcatin B (2), (7S,8S,8'R)-3',4'-dimethoxy-3,4,-methylenedioxylignan-7-ol (3), 1-hydroxy-7-hydroxymethyl-6-methoxyxanthone (4), 5,7-dimethoxy-3',4'-methylenedioxyflavan-3-ol (5), (+)-(3S,4S,6R)-3,6-dihydroxypiperitone (6), protocatechuic acid methyl ester (7) and tyrosol (8) were obtained. All of them had significant neuroprotective activities against glutamate-induced neurotoxicity in primary cultures of rat cortical cells at concentrations ranging from 0.1 microM to 10.0 microM and were comparable to MK-801, a well-known inhibitor of glutamate receptor.
Total synthesis of (-)-talaumidin and (-)-galbelgin.[Pubmed:19408154]
J Asian Nat Prod Res. 2009;11(3):281-7.
( - )-Talaumidin (1) and ( - )-Galbelgin (2) have been synthesized via 4-pentenoic acid as a starting material with the overall yield of about 17.8 and 16.9%, respectively. The key steps include Evans asymmetry anti-aldol reaction, TBS protection, hydroboration, oxidation, Friedel-Crafts arylation, etc.
Stereoselective synthesis of tetrahydrofuran lignans via BF(3) x OEt(2)-promoted reductive deoxygenation/epimerization of cyclic hemiketal: synthesis of (-)-odoratisol C, (-)-futokadsurin A, (-)-veraguensin, (+)-fragransin A(2), (+)-galbelgin, and (+)-talaumidin.[Pubmed:17764190]
Org Lett. 2007 Sep 27;9(20):3965-8.
A versatile route to the synthesis of 2,5-diaryl-3,4-dimethyltetrahydrofuran lignans, (-)-odoratisol C (1), (-)-futokadsurin A (2), (-)-veraguensin (3), (+)-fragransin A2 (4), (+)-Galbelgin (5), and (+)-talaumidin (6), is described. Central to the synthesis of the lignans is BF(3) x OEt(2)-promoted deoxygenation/epimerization of the hemiketal 9a followed by stereoselective reduction of the oxocarbenium ion intermediates 8a,b.


