MethoxyadiantifolineCAS# 115452-09-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115452-09-0 | SDF | Download SDF |
| PubChem ID | 3082725 | Appearance | Yellow powder |
| Formula | C43H52N2O10 | M.Wt | 756.9 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
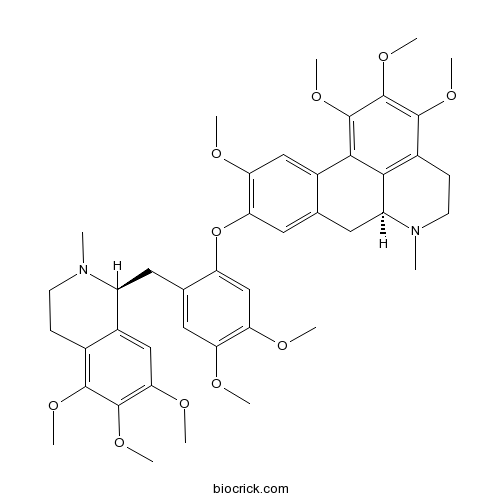
| Chemical Name | (6aS)-9-[4,5-dimethoxy-2-[[(1S)-5,6,7-trimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-1-yl]methyl]phenoxy]-1,2,3,10-tetramethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline | ||
| SMILES | CN1CCC2=C3C1CC4=CC(=C(C=C4C3=C(C(=C2OC)OC)OC)OC)OC5=CC(=C(C=C5CC6C7=CC(=C(C(=C7CCN6C)OC)OC)OC)OC)OC | ||
| Standard InChIKey | NMCGVMFQTRAOOV-KYJUHHDHSA-N | ||
| Standard InChI | InChI=1S/C43H52N2O10/c1-44-14-12-25-28(21-36(49-6)41(52-9)39(25)50-7)29(44)17-24-19-32(46-3)34(48-5)22-31(24)55-35-18-23-16-30-37-26(13-15-45(30)2)40(51-8)43(54-11)42(53-10)38(37)27(23)20-33(35)47-4/h18-22,29-30H,12-17H2,1-11H3/t29-,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methoxyadiantifoline Dilution Calculator

Methoxyadiantifoline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3212 mL | 6.6059 mL | 13.2118 mL | 26.4236 mL | 33.0295 mL |
| 5 mM | 0.2642 mL | 1.3212 mL | 2.6424 mL | 5.2847 mL | 6.6059 mL |
| 10 mM | 0.1321 mL | 0.6606 mL | 1.3212 mL | 2.6424 mL | 3.3029 mL |
| 50 mM | 0.0264 mL | 0.1321 mL | 0.2642 mL | 0.5285 mL | 0.6606 mL |
| 100 mM | 0.0132 mL | 0.0661 mL | 0.1321 mL | 0.2642 mL | 0.3303 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Guttiferone G
Catalog No.:BCN9395
CAS No.:666174-75-0
- [12]-Dehydrogingerdione
Catalog No.:BCN9394
CAS No.:99742-05-9
- 3-O-(6'-O-Galloyl)-β-D-glucopyranosylmaltol
Catalog No.:BCN9393
CAS No.:163397-38-4
- Macarangioside D
Catalog No.:BCN9392
CAS No.:819870-23-0
- Isorhapontin
Catalog No.:BCN9391
CAS No.:32727-29-0
- Magnosalin
Catalog No.:BCN9390
CAS No.:81861-74-7
- 16-Desoxycucurbitacin V
Catalog No.:BCN9389
CAS No.:2081098-85-1
- Sericic acid
Catalog No.:BCN9388
CAS No.:55306-03-1
- Galbelgin
Catalog No.:BCN9387
CAS No.:10569-12-7
- Garcimultiflorone J
Catalog No.:BCN9386
CAS No.:2010216-41-6
- Petiolin F
Catalog No.:BCN9385
CAS No.:1204251-10-4
- Cynanoside F
Catalog No.:BCN9384
CAS No.:1800029-50-8
- Apocynoside I
Catalog No.:BCN9397
CAS No.:358721-31-0
- 10-Dehydrogingerdione
Catalog No.:BCN9398
CAS No.:99742-04-8
- Batatasin I
Catalog No.:BCN9399
CAS No.:51415-00-0
- p-Hydroxybenzaldehyde glucoside
Catalog No.:BCN9400
CAS No.:26993-16-8
- 3β-Isodihydrocadambine
Catalog No.:BCN9401
CAS No.:62014-69-1
- Methoxyeugenol
Catalog No.:BCN9402
CAS No.:6627-88-9
- Platachromone A
Catalog No.:BCN9403
CAS No.:1606149-62-5
- Symphonone I
Catalog No.:BCN9404
CAS No.:1235774-18-1
- Tyramine hydrochloride
Catalog No.:BCN9405
CAS No.:60-19-5
- Mumeose K
Catalog No.:BCN9406
CAS No.:2132384-01-9
- 2,7-Dihydroxyxanthone
Catalog No.:BCN9407
CAS No.:64632-72-0
- 5-Carboxystrictosidine
Catalog No.:BCN9408
CAS No.:34371-47-6
Binding of bisbenzylisoquinoline alkaloids to phosphatidylcholine vesicles and alveolar macrophages: relationship between binding affinity and antifibrogenic potential of these drugs.[Pubmed:1663032]
Exp Lung Res. 1991 Nov-Dec;17(6):1061-77.
A group of bisbenzylisoquinoline alkaloids has been shown to exhibit various degrees of effectiveness in preventing silica-induced fibrosis in animal models. The objective of the present study was to characterize the binding of several of these alkaloids to phosphatidylcholine vesicles and rat alveolar macrophages using fluorometric and equilibrium dialysis methods, respectively. The lipid binding affinity of these alkaloids was found to depend upon several structural factors including hydrophobic substitutions, chiral configurations, and double oxygen bridge-restricted confirmation of the benzylisoquinoline moieties. Tetrandrine, which is a highly effective agent in preventing fibrosis, showed strong binding to both lipid vesicles and alveolar macrophages. In contrast, certain analogues of tetrandrine such as curine and tubocurine, which have little or no effect on silicosis, exhibited only weak binding to lipid vesicles and almost no binding to cells. The moderate binding affinity of fangchinoline to vesicles and cells corresponded to a moderate effectiveness of the compound as an antifibrogenic agent. Methoxyadiantifoline, an alkaloid of unknown antifibrogenic potential, also exhibited high binding affinities for lipid and cells. In conclusion, the results of these studies indicate that alveolar macrophages exhibit large binding capacities for certain members of this class of bisbenzylisoquinoline alkaloids. A positive correlation was observed between binding affinity to alveolar macrophages and the reported antifibrotic potency of these compounds. These data also suggest that the ability of these drugs to interact with alveolar macrophages may be a key step in inhibition of the progression of silica-induced pulmonary disease.
Effects of bisbenzylisoquinoline alkaloids on alveolar macrophages: correlation between binding affinity, inhibitory potency, and antifibrotic potential.[Pubmed:2017754]
Toxicol Appl Pharmacol. 1991 Apr;108(2):242-52.
The Chinese have conducted extensive studies concerning the medicinal properties of plant products. In this investigation the ability of three bisbenzylisoquinoline alkaloids to inhibit particle-induced activation of alveolar macrophages was evaluated and this inhibitory potential was correlated with the ability of those drugs to bind to membrane components. Tetrandrine, i.e., an herbal medicine used as an antifibrotic agent in China, was a potent inhibitor of particle-stimulated oxygen consumption, superoxide release, and hydrogen peroxide secretion by alveolar macrophages. Tetrandrine also exhibited substantial binding affinity for membrane lipids and alveolar macrophages. In contrast, tubocurine, an analogue with little antifibrotic potential, exhibited low binding affinity and had little effect on macrophage activation. Methoxyadiantifoline, an alkaloid of unknown antifibrotic potential, exhibited inhibitory and binding properties similar to those of tetrandrine. The data indicate that a strong relationship exists between the antifibrotic potential of these alkaloids and their ability to bind to alveolar macrophages and inhibit particle-induced activation of these phagocytes. These drugs should serve as useful probes to evaluate the role of alveolar macrophages in pulmonary fibrosis.


