MethyllucidoneCAS# 19956-54-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19956-54-8 | SDF | Download SDF |
| PubChem ID | 21680425 | Appearance | Yellow powder |
| Formula | C16H14O4 | M.Wt | 270.3 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
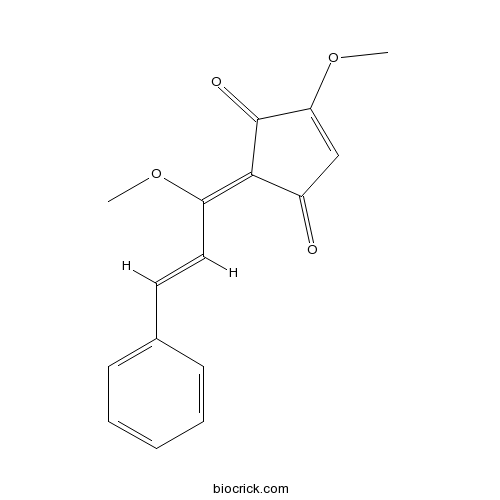
| Chemical Name | (2Z)-4-methoxy-2-[(E)-1-methoxy-3-phenylprop-2-enylidene]cyclopent-4-ene-1,3-dione | ||
| SMILES | COC1=CC(=O)C(=C(C=CC2=CC=CC=C2)OC)C1=O | ||
| Standard InChIKey | FITVJPYUOAZKPN-PBMBQWDMSA-N | ||
| Standard InChI | InChI=1S/C16H14O4/c1-19-13(9-8-11-6-4-3-5-7-11)15-12(17)10-14(20-2)16(15)18/h3-10H,1-2H3/b9-8+,15-13- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Methyllucidone may have a neuroprotective potential via inhibition of neurotoxic microglial activation implicated in neurodegeneration. 2. Methyllucidone can strongly inhibit the growth of human cancer cells and colon tumor xenografted in nude mice, the anti-tumor effects are further confirmed with caspase-3 activation and degradation of PARP. 3. Methyllucidone shows 85% antifungal activity at 50 against the disease wheat leaf rust. |
| Targets | NO | IL Receptor | TNF-α | NF-kB | Akt | p38MAPK | ERK | Caspase | PARP | Antifection |

Methyllucidone Dilution Calculator

Methyllucidone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6996 mL | 18.498 mL | 36.9959 mL | 73.9919 mL | 92.4898 mL |
| 5 mM | 0.7399 mL | 3.6996 mL | 7.3992 mL | 14.7984 mL | 18.498 mL |
| 10 mM | 0.37 mL | 1.8498 mL | 3.6996 mL | 7.3992 mL | 9.249 mL |
| 50 mM | 0.074 mL | 0.37 mL | 0.7399 mL | 1.4798 mL | 1.8498 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.37 mL | 0.7399 mL | 0.9249 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lucidone
Catalog No.:BCN4876
CAS No.:19956-53-7
- NNC 26-9100
Catalog No.:BCC7361
CAS No.:199522-35-5
- 2-Amino-4-chlorobenzothiazole
Catalog No.:BCC8529
CAS No.:19952-47-7
- Veraguensin
Catalog No.:BCN2163
CAS No.:19950-55-1
- Y-33075
Catalog No.:BCC2064
CAS No.:199433-58-4
- 3-Epicabraleadiol
Catalog No.:BCN4875
CAS No.:19942-04-2
- Gymnestrogenin
Catalog No.:BCN7846
CAS No.:19942-02-0
- Kolavenol
Catalog No.:BCN4680
CAS No.:19941-83-4
- Crocatone
Catalog No.:BCN3532
CAS No.:19937-86-1
- Glyurallin A
Catalog No.:BCN7538
CAS No.:199331-36-7
- Dehydroglyasperin C
Catalog No.:BCN6790
CAS No.:199331-35-6
- O6-Benzylguanine
Catalog No.:BCC6485
CAS No.:19916-73-5
- JIB-04
Catalog No.:BCC4548
CAS No.:199596-05-9
- CP 465022 hydrochloride
Catalog No.:BCC7520
CAS No.:199655-36-2
- Ro 61-8048
Catalog No.:BCC7619
CAS No.:199666-03-0
- LY 334370 hydrochloride
Catalog No.:BCC7559
CAS No.:199673-74-0
- Liguiritigenin-7-O-D-apiosyl-4'-O-D-glucoside
Catalog No.:BCN2840
CAS No.:199796-12-8
- 1-Actamido-3,5-dimethyladmantane
Catalog No.:BCC8449
CAS No.:19982-07-1
- Stattic
Catalog No.:BCC1176
CAS No.:19983-44-9
- PD 166793
Catalog No.:BCC2376
CAS No.:199850-67-4
- Isocudraniaxanthone B
Catalog No.:BCN6887
CAS No.:199851-52-0
- RS 127445
Catalog No.:BCC1909
CAS No.:199864-87-4
- DL-Alanyl-DL-Methionine
Catalog No.:BCC8950
CAS No.:1999-43-5
- Chrysoeriol-7-O-glucoside
Catalog No.:BCN3796
CAS No.:19993-32-9
Cyclopentenediones, inhibitors of farnesyl protein transferase and anti-tumor compounds, isolated from the fruit of Lindera erythrocarpa Makino.[Pubmed:16055336]
Bioorg Med Chem. 2005 Nov 15;13(22):6182-7.
Four cyclopentenediones, farnesyl protein transferase inhibitors, and anti-tumor compounds were isolated from the methanolic extract of the fruits of Lindera erythrocarpa Makino (Lauraceae). The structure of the compounds was determined by spectral data including NMR and mass spectrometry, and cyclopentenediones such as methyllinderone (1), Methyllucidone (2), lucidone (3), and linderone (4) were identified by comparing their reported spectral data with that of the literature values. Compounds 1-4 inhibited farnesyl protein transferase with IC50 value of 55.3+/-4.1, 42+/-1.9, 103+/-5.1, and 40+/-3.5 microM, respectively. Isolated compounds also inhibited the growth of various human cancer cell lines in a dose-dependent manner. Especially, Compounds 1 and 2 selectively inhibited the growth of H-ras-transformed rat-2 cell lines in comparison with normal rat-2 cells with a GI50 value of 0.3 and 0.85 microM, respectively. Methyllucidone strongly inhibited the growth of human cancer cells and colon tumor xenografted in nude mice. The anti-tumor effects of the compound were further confirmed with caspase-3 activation and degradation of PARP. The results suggest that Methyllucidone can be a potential anti-cancer agent against H-ras-transformed tumor and will also be a good lead molecule for the development of anti-tumor drug.
Neuroprotective effect of methyl lucidone against microglia-mediated neurotoxicity.[Pubmed:22683871]
Eur J Pharmacol. 2012 Sep 5;690(1-3):4-12.
Excessive microglial activation-mediated neurotoxicity has been implicated in playing a crucial role in the pathogenesis of stroke and neurodegenerative diseases. Therefore, much attention has been paid to therapeutic strategies aimed at suppressing neurotoxic microglial activation. The microglial regulatory mechanism of methyl lucidone, a cyclopentenedione isolated from the stem bark of Lindera erythrocarpa Makino, was investigated in the present study. Methyl lucidone treatment (0.1-10 muM) significantly inhibited lipopolysaccharide (LPS, 100 ng/ml, 24 h)-stimulated nitric oxide (NO) production in a dose-dependent manner in both primary cortical microglia and BV-2 cell line. Moreover, it strongly inhibited LPS-stimulated secretion of pro-inflammatory cytokines, such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-alpha). Methyl lucidone treatment markedly induced down-regulation of LPS-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) through preventing the degradation of the inhibitory protein IkappaBalpha. In addition, phosphorylation of Akt and mitogen-activated protein kinases (MAPKs) such as extracellular signal-regulated kinase (ERK) and p38 kinases were also suppressed by methyl lucidone. The cell viabilities of HT-22 neurons were significantly attenuated by treatment of the conditioned media containing neurotoxic secretary molecules from LPS-stimulated microglia. However, methyl lucidone significantly blocked neuronal cell death induced by microglial conditioned media. These neuroprotective effects of methyl lucidone were also confirmed in a neuron-microglia co-culture system using EGFP-transfected B35 neuroblastoma cell line. Taken together, these results suggest that methyl lucidone may have a neuroprotective potential via inhibition of neurotoxic microglial activation implicated in neurodegeneration.


