Isocudraniaxanthone BCAS# 199851-52-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 199851-52-0 | SDF | Download SDF |
| PubChem ID | 10831150 | Appearance | Powder |
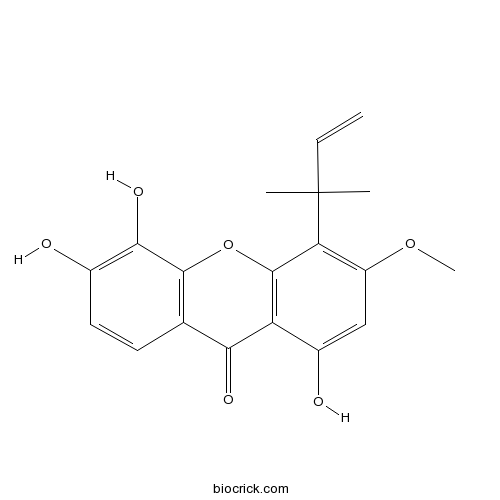
| Formula | C19H18O6 | M.Wt | 342.35 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,5,6-trihydroxy-3-methoxy-4-(2-methylbut-3-en-2-yl)xanthen-9-one | ||
| SMILES | CC(C)(C=C)C1=C(C=C(C2=C1OC3=C(C2=O)C=CC(=C3O)O)O)OC | ||
| Standard InChIKey | PELOBHLTYBBGDO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18O6/c1-5-19(2,3)14-12(24-4)8-11(21)13-15(22)9-6-7-10(20)16(23)17(9)25-18(13)14/h5-8,20-21,23H,1H2,2-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isocudraniaxanthone B may have antimalarial activity. |
| Targets | Sodium Channel | Antifection |

Isocudraniaxanthone B Dilution Calculator

Isocudraniaxanthone B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.921 mL | 14.6049 mL | 29.2099 mL | 58.4197 mL | 73.0247 mL |
| 5 mM | 0.5842 mL | 2.921 mL | 5.842 mL | 11.6839 mL | 14.6049 mL |
| 10 mM | 0.2921 mL | 1.4605 mL | 2.921 mL | 5.842 mL | 7.3025 mL |
| 50 mM | 0.0584 mL | 0.2921 mL | 0.5842 mL | 1.1684 mL | 1.4605 mL |
| 100 mM | 0.0292 mL | 0.146 mL | 0.2921 mL | 0.5842 mL | 0.7302 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PD 166793
Catalog No.:BCC2376
CAS No.:199850-67-4
- Stattic
Catalog No.:BCC1176
CAS No.:19983-44-9
- 1-Actamido-3,5-dimethyladmantane
Catalog No.:BCC8449
CAS No.:19982-07-1
- Liguiritigenin-7-O-D-apiosyl-4'-O-D-glucoside
Catalog No.:BCN2840
CAS No.:199796-12-8
- LY 334370 hydrochloride
Catalog No.:BCC7559
CAS No.:199673-74-0
- Ro 61-8048
Catalog No.:BCC7619
CAS No.:199666-03-0
- CP 465022 hydrochloride
Catalog No.:BCC7520
CAS No.:199655-36-2
- JIB-04
Catalog No.:BCC4548
CAS No.:199596-05-9
- Methyllucidone
Catalog No.:BCN4877
CAS No.:19956-54-8
- Lucidone
Catalog No.:BCN4876
CAS No.:19956-53-7
- NNC 26-9100
Catalog No.:BCC7361
CAS No.:199522-35-5
- 2-Amino-4-chlorobenzothiazole
Catalog No.:BCC8529
CAS No.:19952-47-7
- RS 127445
Catalog No.:BCC1909
CAS No.:199864-87-4
- DL-Alanyl-DL-Methionine
Catalog No.:BCC8950
CAS No.:1999-43-5
- Chrysoeriol-7-O-glucoside
Catalog No.:BCN3796
CAS No.:19993-32-9
- UCL 1684
Catalog No.:BCC7016
CAS No.:199934-16-2
- CVT-313
Catalog No.:BCC1503
CAS No.:199986-75-9
- ALX 5407 hydrochloride
Catalog No.:BCC7168
CAS No.:200006-08-2
- Benzyl 4-bromophenyl ketone
Catalog No.:BCC8868
CAS No.:2001-29-8
- Valinomycin
Catalog No.:BCC7671
CAS No.:2001-95-8
- H-Arg(Pbf)-OH
Catalog No.:BCC2866
CAS No.:200115-86-2
- H-D-Arg(Pbf)-OH
Catalog No.:BCC2872
CAS No.:200116-81-0
- SKF 38393 hydrobromide
Catalog No.:BCC6848
CAS No.:20012-10-6
- Boc-Arg(Pbf)-OH
Catalog No.:BCC3066
CAS No.:200124-22-7
Antimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii.[Pubmed:15474559]
Life Sci. 2004 Nov 5;75(25):3077-85.
The antimalarial activity of 22 xanthones against chloroquino-resistant strains of Plasmodium falciparum was evaluated. Natural caloxanthone C (1), demethylcalabaxanthone (2), calothwaitesixanthone (3), calozeyloxanthone (4), dombakinaxanthone (5), macluraxanthone (6), and 6-deoxy-gamma-mangostin (7) were isolated from Calophyllum caledonicum. 1,6-dihydroxyxanthone (8), pancixanthone A (9), Isocudraniaxanthone B (10), isocudraniaxanthone A (11), 2-deprenylrheediaxanthone B (12) and 1,4,5-trihydroxyxanthone (13) were isolated from Garcinia vieillardii. Moreover, synthetic compounds (14-22) are analogues or intermediates of xanthones purified from Calophyllum caledonicum (Oger J.M., Morel C., Helesbeux J.J., Litaudon M., Seraphin D., Dartiguelongue C., Larcher G., Richomme P., Duval O. 2003. First 2-Hydroxy-3-Methylbut-3-Enyl substituted xanthones isolated from Plants: structure elucidation, synthesis and antifungal activity. Natural Product Research 17(3), 195-199; Helesbeux J.J., Duval O., Dartiguelongue C., Seraphin D., Oger J.M., Richomme P., 2004. Synthesis of 2-hydroxy-3-methylbut-3-enyl substituted coumarins and xanthones as natural products. Application of the Schenck ene reaction of singlet oxygen with ortho-prenylphenol precursors. Tetrahedron 60(10), 2293-2300). The relationship between antimalarial activity and molecular structure of xanthones has also been explored. The most potent xanthones (2), (3) and (7) (IC50 = c.a. 1.0 microg/mL) are 1,3,7 trioxygenated and prenylated on the positions 2 and 8.


