O6-BenzylguaninePotent MGMT inhibitor CAS# 19916-73-5 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19916-73-5 | SDF | Download SDF |
| PubChem ID | 4578 | Appearance | Powder |
| Formula | C12H11N5O | M.Wt | 241.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >11.3mg/mL in DMSO | ||
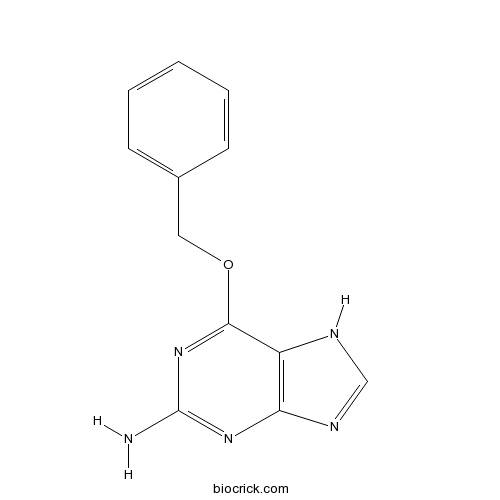
| Chemical Name | 6-phenylmethoxy-7H-purin-2-amine | ||
| SMILES | C1=CC=C(C=C1)COC2=NC(=NC3=C2NC=N3)N | ||
| Standard InChIKey | KRWMERLEINMZFT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H11N5O/c13-12-16-10-9(14-7-15-10)11(17-12)18-6-8-4-2-1-3-5-8/h1-5,7H,6H2,(H3,13,14,15,16,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

O6-Benzylguanine Dilution Calculator

O6-Benzylguanine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1459 mL | 20.7297 mL | 41.4594 mL | 82.9187 mL | 103.6484 mL |
| 5 mM | 0.8292 mL | 4.1459 mL | 8.2919 mL | 16.5837 mL | 20.7297 mL |
| 10 mM | 0.4146 mL | 2.073 mL | 4.1459 mL | 8.2919 mL | 10.3648 mL |
| 50 mM | 0.0829 mL | 0.4146 mL | 0.8292 mL | 1.6584 mL | 2.073 mL |
| 100 mM | 0.0415 mL | 0.2073 mL | 0.4146 mL | 0.8292 mL | 1.0365 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
O6-Benzylguanine (BG) is a potent inhibitor of MGMT and an antineoplastic agent [1].
O6-methylguanine DNA methyltransferase (MGMT, also known as O6-Alkylguanine-DNA alkyltransferase, or AGT) is a DNA repair protein that removes the alkyl group located on the O6-position of guanine from DNA and restores DNA integrity. MGMT is alkylated and irreversibly inactivated.
O6-Benzylguanine is a potent MGMT inhibitor. In HT29 cells, O6-Benzylguanine significantly reduced MGMT stability and the affinity of MGMT for DNA, increased its sensitivity to proteases. O6-Benzylguanine also increased the cytotoxic effect of alkylating agent BCNU [1]. In SF767 cells, BG (25 μM for 1 h) reduced >95% of MGMT activity, while 33% of the activity recovered within 24 h. When cells pretreated with BG (2.5 μM for 24 h) followed by the same low-dose treatment for 24 h completely depleted MGMT activity [2]. In HCT116 and HCT15 cells, treatment with BG and BCNU inactivated MGMT and arrested 70-80% of cells in G2/M phase in response to the DNA damages induced by BCNU [3].
In nude mice bearing SF767 human brain tumor xenografts, treatment with O6-Benzylguanine prior to BCNU significantly inhibited tumor growth as compared to BCNU alone [1]. In xenograft SF767 tumors, pre- and post-treatments (8 mg/kg over 24 h) combined with an i.p. bolus dose (80 mg/kg) of BG inhibited >95% of MGMT activity [2].
References:
[1]. Dolan ME, Pegg AE. O6-benzylguanine and its role in chemotherapy. Clin Cancer Res, 1997, 3(6): 837-847.
[2]. Kreklau EL, Kurpad C, Williams DA, et al. Prolonged inhibition of O(6)-methylguanine DNA methyltransferase in human tumor cells by O(6)-benzylguanine in vitro and in vivo. J Pharmacol Exp Ther, 1999, 291(3): 1269-1275.
[3]. Yan L, Donze JR, Liu L. Inactivated MGMT by O6-benzylguanine is associated with prolonged G2/M arrest in cancer cells treated with BCNU. Oncogene, 2005, 24(13): 2175-2183.
- Boc-D-N-Me-Ala-OH
Catalog No.:BCC3211
CAS No.:19914-38-6
- Enniatin B1
Catalog No.:BCN4853
CAS No.:19914-20-6
- Futoenone
Catalog No.:BCN6408
CAS No.:19913-01-0
- Chamigrenal
Catalog No.:BCN7847
CAS No.:19912-84-6
- Furanodiene
Catalog No.:BCN6454
CAS No.:19912-61-9
- T 98475
Catalog No.:BCC7395
CAS No.:199119-18-1
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- H-Phe(4-Me)-OH
Catalog No.:BCC3270
CAS No.:1991-87-3
- Maackiain
Catalog No.:BCN1236
CAS No.:19908-48-6
- Bakkenolide A
Catalog No.:BCN5402
CAS No.:19906-72-0
- Dihydromethysticin
Catalog No.:BCN2476
CAS No.:19902-91-1
- Syringaresinol diacetate
Catalog No.:BCN4874
CAS No.:1990-77-8
- Dehydroglyasperin C
Catalog No.:BCN6790
CAS No.:199331-35-6
- Glyurallin A
Catalog No.:BCN7538
CAS No.:199331-36-7
- Crocatone
Catalog No.:BCN3532
CAS No.:19937-86-1
- Kolavenol
Catalog No.:BCN4680
CAS No.:19941-83-4
- Gymnestrogenin
Catalog No.:BCN7846
CAS No.:19942-02-0
- 3-Epicabraleadiol
Catalog No.:BCN4875
CAS No.:19942-04-2
- Y-33075
Catalog No.:BCC2064
CAS No.:199433-58-4
- Veraguensin
Catalog No.:BCN2163
CAS No.:19950-55-1
- 2-Amino-4-chlorobenzothiazole
Catalog No.:BCC8529
CAS No.:19952-47-7
- NNC 26-9100
Catalog No.:BCC7361
CAS No.:199522-35-5
- Lucidone
Catalog No.:BCN4876
CAS No.:19956-53-7
- Methyllucidone
Catalog No.:BCN4877
CAS No.:19956-54-8
New insights into estrogenic regulation of O(6)-methylguanine DNA-methyltransferase (MGMT) in human breast cancer cells: Co-degradation of ER-alpha and MGMT proteins by fulvestrant or O(6)-benzylguanine indicates fresh avenues for therapy.[Pubmed:27845303]
J Biomed Res. 2016 Sep;30(5):393-410.
Endocrine therapy using estrogen receptor-alpha (ER-alpha) antagonists for attenuating horm2one-driven cell proliferation is a major treatment modality for breast cancers. To exploit any DNA repair deficiencies associated with endocrine therapy, we investigated the functional and physical interactions of ER-alpha with O(6)-methylguanine DNA methyltransferase (MGMT), a unique DNA repair protein that confers tumor resistance to various anticancer alkylating agents. The ER-alpha -positive breast cancer cell lines (MCF-7, T47D) and ER- negative cell lines (MDAMB-468, MDAMB-231), and established inhibitors of ER-alpha and MGMT, namely, ICI-182,780 (Faslodex) and O(6)-benzylguanine, respectively, were used to study MGMT- ER interactions. The MGMT gene promoter was found to harbor one full and two half estrogen-responsive elements (EREs) and two antioxidant-responsive elements (AREs). MGMT expression was upregulated by estrogen, downregulated by tamoxifen in Western blot and promoter-linked reporter assays. Similarly, both transient and stable transfections of Nrf-2 (nuclear factor-erythroid 2-related factor-2) increased the levels of MGMT protein and activity 3 to 4-fold reflecting novel regulatory nodes for this drug-resistance determinant. Of the different ER-alpha antagonists tested, the pure anti-estrogen fulvestrant was most potent in inhibiting the MGMT activity in a dose, time and ER-alpha dependent manner, similar to O(6)-benzylguanine. Interestingly, fulvestrant exposure led to a degradation of both ER-alpha and MGMT proteins and O(6)-benzylguanine also induced a specific loss of ER-alpha and MGMT proteins in MCF-7 and T47D breast cancer cells with similar kinetics. Immunoprecipitation revealed a specific association of ER-alpha and MGMT proteins in breast cancer cells. Furthermore, silencing of MGMT gene expression triggered a decrease in the levels of both MGMT and ER-alpha proteins. The involvement of proteasome in the drug-induced degradation of both proteins was also demonstrated. Fulvestrant enhanced the cytotoxicity of MGMT-targeted alkylating agents, namely, temozolomide and BCNU by 3 to 4-fold in ER-alpha positive cells, but not in ER-negative cells. We conclude that MGMT and ER-alpha proteins exist as a complex and are co-targeted for ubiquitin-conjugation and subsequent proteasomal degradation. The findings offer a clear rationale for combining alkylating agents with endocrine therapy.
Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O6-benzylguanine to brain tumors.[Pubmed:25247850]
ACS Nano. 2014 Oct 28;8(10):10383-95.
Resistance to temozolomide (TMZ) based chemotherapy in glioblastoma multiforme (GBM) has been attributed to the upregulation of the DNA repair protein O(6)-methylguanine-DNA methyltransferase (MGMT). Inhibition of MGMT using O(6)-benzylguanine (BG) has shown promise in these patients, but its clinical use is hindered by poor pharmacokinetics that leads to unacceptable toxicity. To improve BG biodistribution and efficacy, we developed superparamagnetic iron oxide nanoparticles (NP) for targeted convection-enhanced delivery (CED) of BG to GBM. The nanoparticles (NPCP-BG-CTX) consist of a magnetic core coated with a redox-responsive, cross-linked, biocompatible chitosan-PEG copolymer surface coating (NPCP). NPCP was modified through covalent attachment of BG and tumor targeting peptide chlorotoxin (CTX). Controlled, localized BG release was achieved under reductive intracellular conditions and NPCP-BG-CTX demonstrated proper trafficking of BG in human GBM cells in vitro. NPCP-BG-CTX treated cells showed a significant reduction in MGMT activity and the potentiation of TMZ toxicity. In vivo, CED of NPCP-BG-CTX produced an excellent volume of distribution (Vd) within the brain of mice bearing orthotopic human primary GBM xenografts. Significantly, concurrent treatment with NPCP-BG-CTX and TMZ showed a 3-fold increase in median overall survival in comparison to NPCP-CTX/TMZ treated and untreated animals. Furthermore, NPCP-BG-CTX mitigated the myelosuppression observed with free BG in wild-type mice when administered concurrently with TMZ. The combination of favorable physicochemical properties, tumor cell specific BG delivery, controlled BG release, and improved in vivo efficacy demonstrates the great potential of these NPs as a treatment option that could lead to improved clinical outcomes.
Evaluation of O6-Benzylguanine-Potentiated Topical Carmustine for Mycosis Fungoides: A Phase 1-2 Clinical Trial.[Pubmed:28199478]
JAMA Dermatol. 2017 May 1;153(5):413-420.
Importance: In a phase 1 trial, single-dose O6-Benzylguanine with topical carmustine for patients with early stage (stage IA through stage IIA) cutaneous T-cell lymphoma, mycosis fungoides (MF) type, resulted in clinical responses proportional to inhibition of O6-alkylguanine-DNA alkyltransferase activity, but a maximum tolerated dose (MTD) was not reached. Objective: To determine whether dose escalation of carmustine in combination with dual-dose O6-Benzylguanine to prolong alkyltransferase inhibition could reach an MTD. Design, Setting, and Participants: A single-arm, phase 1-2 clinical trial conducted at a university teaching hospital enrolled 17 adults with stage IA through stage IIA cutaneous T-cell lymphoma, MF type, to evaluate treatment using topical carmustine plus 2 subsequent daily doses of intravenous O6-Benzylguanine, administered every 2 weeks for up to 24 weeks (12 cycles). All patients who received treatment were included in an intent-to-treat analysis of the response rate. The study was conducted from February 17, 2010, to April 8, 2014. Data analysis was performed from May 1, 2014, to December 1, 2015. Interventions: Topical carmustine and intravenous O6-Benzylguanine. Main Outcomes and Measures: Clinical disease response was assessed by the Severity-Weighted Assessment Tool (score range, 0-400; higher score indicates worse disease). Safety data were acquired by review of adverse events at study visits. Results: Of the 17 patients enrolled, 12 (71%) were men; mean (SD) age was 45.2 (14.6) years. There were 7 complete responses and 8 partial responses to combination carmustine and O6-Benzylguanine treatment. The overall clinical response rate was 88%, with a mean (SD) duration of complete response of 14.43 (6.6) months. The MTD was 20 mg of carmustine applied once in combination with 2 daily doses of 120 mg/m2 of O6-Benzylguanine. Most adverse events (112 [67%]) were grade I. Of 15 patients with dermatitis, 5 individuals (33%) demonstrated grade II dermatitis that was unresponsive to topical corticosteroid therapy. The dermatitis was characterized by high levels of macrophage activation, and clearance was associated with vitamin D3 administration. Conclusions and Relevance: Compared with single-dose O6-Benzylguanine and carmustine, dual-dose O6-Benzylguanine resulted in higher overall response rates and reduced total carmustine doses but was associated with more cutaneous adverse events. The MTD for dual-dose O6-Benzylguanine plus carmustine was also ascertained. Trial Registration: clinicaltrials.gov Identifier: NCT00961220.
iRGD-mediated core-shell nanoparticles loading carmustine and O(6)-benzylguanine for glioma therapy.[Pubmed:27646474]
J Drug Target. 2017 Mar;25(3):235-246.
iRGD (internalizing RGD) with high affinity to alphanu integrins was reported to enhance tumor penetrability by binding to neuropilin-1 (NRP-1). Based on our previous study, chitosan surface-modified poly (lactide-co-glycolides) nanoparticles (PLGA/CS NPs), loaded with carmustine (BCNU) and its sensitizer (O(6)-benzylguanine, BG) showed stronger anti-tumor effect than free drugs. In present study, PLGA/CS NPs (NPs) with core-shell structure were prepared and modified with iRGD or mPEG. F98, C6 or U87 cell lines with different receptors levels were selected for in vitro and in vivo studies. After administration of iRGD-mediated NPs, including iRGD-modified NPs (iRGD-NPs) and co-administration of iRGD and NPs (iRGD + NPs), their effects on glioma were compared with NPs. iRGD-NPs showed stronger cytotoxicity and cellular uptake than other groups. iRGD-NPs and iRGD + NPs displayed deeper tumor penetration and stronger anti-invasion effect on three dimensional (3D) glioma spheroids than NPs. On F98 glioma-bearing mice model, iRGD-mediated NPs showed enhanced crossing BBB ability and brain tumor accumulation levels. Correspondingly, the median survival time of iRGD + NPs, iRGD-NPs and NPs groups were 58, 49 and 34.5 days, respectively. Present studies supported the iRGD-mediated strategy to improve the efficacy of antitumor drug delivery system. Importantly, co-administration of iRGD may be a greater way over the conjugation of iRGD.


