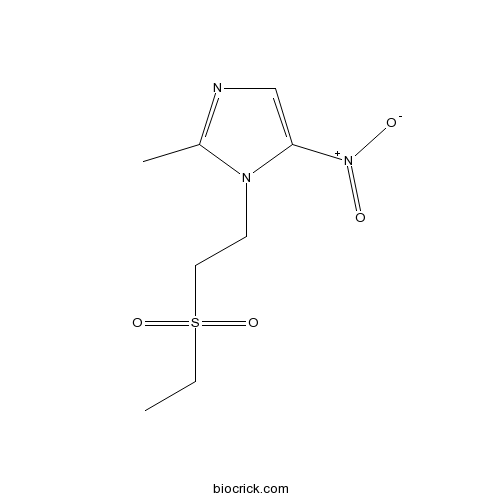
TinidazoleCAS# 19387-91-8 |
- Dexmedetomidine HCl
Catalog No.:BCC4347
CAS No.:145108-58-3
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Isoprenaline HCl
Catalog No.:BCC4328
CAS No.:51-30-9
- Metoprolol Tartrate
Catalog No.:BCC4330
CAS No.:56392-17-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19387-91-8 | SDF | Download SDF |
| PubChem ID | 5479 | Appearance | Powder |
| Formula | C8H13N3O4S | M.Wt | 247.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (202.21 mM) H2O : 3.33 mg/mL (13.47 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1-(2-ethylsulfonylethyl)-2-methyl-5-nitroimidazole | ||
| SMILES | CCS(=O)(=O)CCN1C(=NC=C1[N+](=O)[O-])C | ||
| Standard InChIKey | HJLSLZFTEKNLFI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H13N3O4S/c1-3-16(14,15)5-4-10-7(2)9-6-8(10)11(12)13/h6H,3-5H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tinidazole is a synthesized imidazole derivative used in antiprotozoal treatment with antiamebic and antibacterial properties.
Target: Antibacterial
Tinidazole is a 5-nitroimidazole active in vitro against a wide variety of anaerobic bacteria and protozoa. Tinidazole is an effective treatment against anaerobic microorganisms based on its pharmacokinetic characteristics (C(max) 51 microg/ml, t(1/2) 12.5 h) and its excellent in vitro activity. Its long half-life allows once a day regimens. Tinidazole is as effective as metronidazole in the treatment of infections caused by T. vaginalis, giardiasis and amebiasis and bacterial vaginosis, malaria, odontogenic infections, anaerobic bacterial infections (pelvic inflammatory disease, diabetic foot), surgical prophylaxis (abdominal and hysterectomy) and Helicobacter pylori eradication.
Tinidazole has recently been resurrected and FDA approved for trichomoniasis and BV in the USA and is being restudied as an alternative to metronidazole for BV. In vitro antimicrobial activity and pharmacokinetics studies indicate that when compared directly with metronidazole, tinidazole has minor but possibly relevant antimicrobial as well as pharmacokinetic advantages. References: | |||||

Tinidazole Dilution Calculator

Tinidazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0442 mL | 20.2208 mL | 40.4416 mL | 80.8832 mL | 101.1041 mL |
| 5 mM | 0.8088 mL | 4.0442 mL | 8.0883 mL | 16.1766 mL | 20.2208 mL |
| 10 mM | 0.4044 mL | 2.0221 mL | 4.0442 mL | 8.0883 mL | 10.1104 mL |
| 50 mM | 0.0809 mL | 0.4044 mL | 0.8088 mL | 1.6177 mL | 2.0221 mL |
| 100 mM | 0.0404 mL | 0.2022 mL | 0.4044 mL | 0.8088 mL | 1.011 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tinidazole is a synthesized imidazole derivative used in antiprotozoal treatment with antiamebic and antibacterial properties.
- Cortistatin 14
Catalog No.:BCC6010
CAS No.:193829-96-8
- SB 242235
Catalog No.:BCC4171
CAS No.:193746-75-7
- Aminoguanidine hydrochloride
Catalog No.:BCC6795
CAS No.:1937-19-5
- 8beta-Methoxyatractylenolide I
Catalog No.:BCN7594
CAS No.:193694-24-5
- Methyl 4-hydroxycinnamate
Catalog No.:BCN4014
CAS No.:19367-38-5
- TAS 301
Catalog No.:BCC6214
CAS No.:193620-69-8
- BRL-15572
Catalog No.:BCC5065
CAS No.:193611-72-2
- SB 216641 hydrochloride
Catalog No.:BCC6987
CAS No.:193611-67-5
- Terrestrosin K
Catalog No.:BCN2935
CAS No.:193605-07-1
- GB 1b
Catalog No.:BCN7385
CAS No.:19360-72-6
- Calcifediol
Catalog No.:BCC4949
CAS No.:19356-17-3
- SB 239063
Catalog No.:BCC1923
CAS No.:193551-21-2
- Fmoc-β-Homo-Leu-OH
Catalog No.:BCC2631
CAS No.:193887-44-4
- 3-Phenyl-1,2-dihydroacenaphthylene-1,2-diol
Catalog No.:BCN7178
CAS No.:193892-33-0
- SC 19220
Catalog No.:BCC6968
CAS No.:19395-87-0
- Fmoc-ß-HoAla-OH
Catalog No.:BCC3225
CAS No.:193954-26-6
- Fmoc- ß-HoIle-OH
Catalog No.:BCC3237
CAS No.:193954-27-7
- Fmoc-β-HoPhe-OH
Catalog No.:BCC3241
CAS No.:193954-28-8
- 10-O-Vanilloylaucubin
Catalog No.:BCN1185
CAS No.:193969-08-3
- Cedrelopsin
Catalog No.:BCN7687
CAS No.:19397-28-5
- 6,19-Dihydroxyurs-12-en-3-oxo-28-oic acid
Catalog No.:BCN1513
CAS No.:194027-11-7
- 3-Amino-4-methylbenzamide
Catalog No.:BCC8614
CAS No.:19406-86-1
- Dihydrocapsaicin
Catalog No.:BCN1017
CAS No.:19408-84-5
- Rivulobirin B
Catalog No.:BCN1186
CAS No.:194145-29-4
Randomized, double-blind, comparative study of oral metronidazole and tinidazole in treatment of bacterial vaginosis.[Pubmed:28066102]
Indian J Pharmacol. 2016 Nov-Dec;48(6):654-658.
OBJECTIVE: To compare the efficacy and tolerability of oral metronidazole and Tinidazole in patients with bacterial vaginosis (BV) using Amsel's criteria. PATIENTS AND METHODS: This was a randomized double-blind study, conducted by the Departments of Pharmacology and Gynecology of a tertiary care teaching hospital. Patients diagnosed with BV received either tablet metronidazole 500 mg twice daily for 5 days or tablet Tinidazole 500 mg once daily + one placebo for 5 days and instructed to come for follow-up at the 1(st) week and 4(th) week. They were categorized as cured, partially cured, and not cured based on Amsel's criteria at the end of the study and compared between two groups using Chi-square test. RESULTS: A total 120 women were enrolled in the study, of which 114 completed the study. The treatment arms were comparable. The cure rate with low-dose Tinidazole was significantly more compared to metronidazole at 4(th) week (P = 0.0013), but not at 1(st) week (P = 0.242). The adverse drug reactions were less with Tinidazole compared to metronidazole. CONCLUSION: Tinidazole at lower dose offers a better efficacy than metronidazole in long-term cure rates and in preventing relapses with better side effect profile.
A meta-analysis of the efficacy of albendazole compared with tinidazole as treatments for Giardia infections in children.[Pubmed:26476393]
Acta Trop. 2016 Jan;153:120-7.
Metronidazole is frequently used against Giardia infection; however, it has been associated with significant failure rates in clearing parasites from the gut; additionally, as it should be taken for 5 to 10 days, it is associated with poor compliance, probably due to side effects. Other drugs, including Tinidazole (TNZ) and albendazole (ABZ) have been included in the antigiardial armamentarium. Our aim was to assess the efficacy of ABZ compared with TNZ in Giardia infections in children. A systematic review and a meta-analysis were carried out. PubMed, Medline, EMBASE, CENTRAL, and LILACS were searched electronically until February 2015. Also relevant journals and references of studies included therein were hand-searched for randomised controlled trials (RCTs). The meta-analysis was limited to RCTs evaluating the use of ABZ compared with TNZ in children with Giardia infection. The assessed outcome was parasitological efficacy. Prediction intervals (PI) were computed to better express uncertainties in the effect estimates. Five RCTs including 403 children were included. Overall, TNZ significantly outperformed ABZ without differences between subgroups defined by ABZ dosages [relative risk, (RR) 1.61 (95% CI): (1.40-1.85); P<0.0001]. The 95% prediction interval range is 1.28-2.02. There was no significant heterogeneity (I(2)=0%; Q-test of heterogeneity P=0.4507. The number-needed-to-treat, the average number of patients who need to be treated with TNZ to gain one additional good outcome as compared with ABZ was 4, 95% CI: 3-5. Our results show that TNZ outperforms ABZ in the treatment of Giardia infections in children from developing countries.
Development of microbial trigger based oral formulation of Tinidazole and its Gamma Scintigraphy Evaluation: A promising tool against anaerobic microbes associated GI problems.[Pubmed:27108116]
Eur J Pharm Sci. 2016 Jun 30;89:94-104.
Tinidazole is a versatile anti-amoebic and anti-anaerobic drug used in treatment of intestinal infection. The aim of present study was to develop and evaluate a guar gum based novel target release Tinidazole matrix tablet in animal models and healthy human volunteer using Gamma Scintigraphy technique. Anti-anaerobic and anti-protozoal activity of the developed formulation was studied in vitro against Bacteroides fragilis and Dentamoeba fragilis. Tinidazole was successful radiolabelled with (99m)Tc-pertechnetate using stannous chloride as a reducing agent and stable up to 24h in normal saline and serum. Radiolabeled formulation was evaluated in 6 Newzealand white rabbits by gamma Scintigraphy in static manner up to 24h for its retention in gastrointestinal tract (GIT). Similar set of study was conducted in 12 healthy human volunteers for similar objective Scintigraphy images of healthy human volunteer showed retention of optimized formulations in stomach up to 60min, from where it moved to duodenum further and reached ileum in around 5h. However, initiation of drug release was observed from intestine at 7h. Complete dissociation and release of drug was observed at 24h in colon due to anaerobic microbial rich environment. Results drawn from Scintigraphy images indicate that radiolabeled (99m)Tc-Tinidazole tablet transit through upper part of GI without disintegration. Hence the developed matrix tablet may have a role in treatment of intestinal infection caused by anaerobic bacteria.


