DihydrocapsaicinCAS# 19408-84-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19408-84-5 | SDF | Download SDF |
| PubChem ID | 107982 | Appearance | White powder |
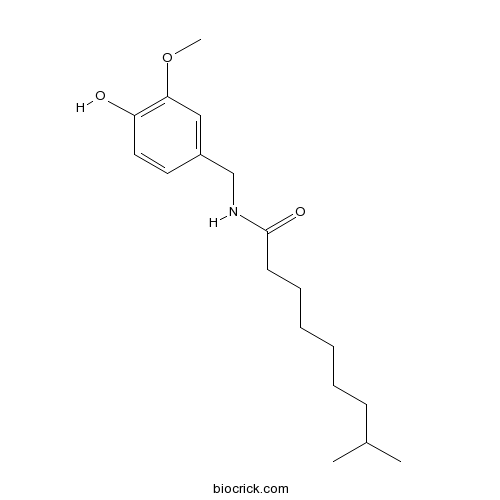
| Formula | C18H29NO3 | M.Wt | 307.43 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | 8-Methyl N-vanillylnonamide | ||
| Solubility | Soluble in dichloromethane and methan | ||
| Chemical Name | N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnonanamide | ||
| SMILES | CC(C)CCCCCCC(=O)NCC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | XJQPQKLURWNAAH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H29NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h10-12,14,20H,4-9,13H2,1-3H3,(H,19,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dihydrocapsaicin, a potential inducer of autophagy, has cytotoxic activity. It has anti-atherogenic activity, can reduce the susceptibility of low-density lipoprotein (LDL) to oxidation. Dihydrocapsaicin treatment depletes peptidergic nerve fibers of substance P and alters mast cell density in the respiratory tract of neonatal sheep. |
| Targets | LDL | Caspase | p53 | ROS | Autophagy | LC3-II |
| In vitro | Effects of capsaicin, dihydrocapsaicin, and curcumin on copper-induced oxidation of human serum lipids.[Pubmed: 16910741 ]J Agric Food Chem. 2006 Aug 23;54(17):6436-9.The oxidation of low-density lipoprotein (LDL) is believed to be the initiating factor for the development and progression of atherosclerosis. The active ingredients of spices such as chili and turmeric (capsaicin and curcumin, respectively) have been shown to reduce the susceptibility of LDL to oxidation.
|
| In vivo | Dihydrocapsaicin treatment depletes peptidergic nerve fibers of substance P and alters mast cell density in the respiratory tract of neonatal sheep.[Pubmed: 10967206]Regul Pept. 2000 Jul 28;91(1-3):97-106.
|
| Cell Research | Dihydrocapsaicin (DHC), a saturated structural analog of capsaicin, induces autophagy in human cancer cells in a catalase-regulated manner.[Pubmed: 18818525]Autophagy. 2008 Nov;4(8):1009-19.Although capsaicin, a pungent component of red pepper, is known to induce apoptosis in several types of cancer cells, the mechanisms underlying capsaicin-induced cytotoxicity are unclear.
|
| Structure Identification | J Pharm Biomed Anal. 2014 Oct 25;103C:59-66.A validated HPLC-FLD method for analysis of intestinal absorption and metabolism of capsaicin and dihydrocapsaicin in the rat.[Pubmed: 25462121]
|

Dihydrocapsaicin Dilution Calculator

Dihydrocapsaicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2528 mL | 16.2639 mL | 32.5277 mL | 65.0555 mL | 81.3193 mL |
| 5 mM | 0.6506 mL | 3.2528 mL | 6.5055 mL | 13.0111 mL | 16.2639 mL |
| 10 mM | 0.3253 mL | 1.6264 mL | 3.2528 mL | 6.5055 mL | 8.1319 mL |
| 50 mM | 0.0651 mL | 0.3253 mL | 0.6506 mL | 1.3011 mL | 1.6264 mL |
| 100 mM | 0.0325 mL | 0.1626 mL | 0.3253 mL | 0.6506 mL | 0.8132 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Amino-4-methylbenzamide
Catalog No.:BCC8614
CAS No.:19406-86-1
- 6,19-Dihydroxyurs-12-en-3-oxo-28-oic acid
Catalog No.:BCN1513
CAS No.:194027-11-7
- Cedrelopsin
Catalog No.:BCN7687
CAS No.:19397-28-5
- 10-O-Vanilloylaucubin
Catalog No.:BCN1185
CAS No.:193969-08-3
- Fmoc-β-HoPhe-OH
Catalog No.:BCC3241
CAS No.:193954-28-8
- Fmoc- ß-HoIle-OH
Catalog No.:BCC3237
CAS No.:193954-27-7
- Fmoc-ß-HoAla-OH
Catalog No.:BCC3225
CAS No.:193954-26-6
- SC 19220
Catalog No.:BCC6968
CAS No.:19395-87-0
- 3-Phenyl-1,2-dihydroacenaphthylene-1,2-diol
Catalog No.:BCN7178
CAS No.:193892-33-0
- Fmoc-β-Homo-Leu-OH
Catalog No.:BCC2631
CAS No.:193887-44-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Cortistatin 14
Catalog No.:BCC6010
CAS No.:193829-96-8
- Rivulobirin B
Catalog No.:BCN1186
CAS No.:194145-29-4
- Isosaponarin
Catalog No.:BCN2279
CAS No.:19416-87-6
- Isohyenanchin
Catalog No.:BCN1187
CAS No.:19417-00-6
- N-Acetyl-5,6-dehydrololine
Catalog No.:BCN2007
CAS No.:194205-01-1
- Selinidin
Catalog No.:BCN2711
CAS No.:19427-82-8
- Curcumenol
Catalog No.:BCN3522
CAS No.:19431-84-6
- Ac-D-Ala-OH
Catalog No.:BCC3196
CAS No.:19436-52-3
- Benzyl Caffeate
Catalog No.:BCC5100
CAS No.:107843-77-6
- CL-387785 (EKI-785)
Catalog No.:BCC6436
CAS No.:194423-06-8
- PD168393
Catalog No.:BCC1157
CAS No.:194423-15-9
- Taberpsychine
Catalog No.:BCN4047
CAS No.:19452-84-7
- 24-Norhopa-4(23),22(29)-diene-3β,6β-diol
Catalog No.:BCN1512
CAS No.:194613-74-6
Dihydrocapsaicin (DHC), a saturated structural analog of capsaicin, induces autophagy in human cancer cells in a catalase-regulated manner.[Pubmed:18818525]
Autophagy. 2008 Nov;4(8):1009-19. Epub 2008 Nov 2.
Although capsaicin, a pungent component of red pepper, is known to induce apoptosis in several types of cancer cells, the mechanisms underlying capsaicin-induced cytotoxicity are unclear. Here, we showed that Dihydrocapsaicin (DHC), an analog of capsaicin, is a potential inducer of autophagy. DHC was more cytotoxic than capsaicin in HCT116, MCF-7 and WI38 cell lines. Capsaicin and DHC did not affect the sub-G(1) apoptotic peak, but induced G(0)/G(1) arrest in HCT116 and MCF-7 cells. DHC caused the artificial autophagosome marker GFP-LC3 to redistribute and upregulated expression of autophagy-related proteins. Blocking of autophagy by 3-methyladenine (3MA) as well as siRNA Atg5 induced a high level of caspase-3 activation. Although pretreatment with zVAD completely inhibited caspase-3 activation by 3MA, it did not prevent cell death. DHC-induced autophagy was enhanced by zVAD pretreatment, as shown by increased accumulation of LC3-II protein. DHC attenuated basal ROS levels through catalase induction; this effect was enhanced by antioxidants, which increased both LC3-II expression and caspase-3 activation. The catalase inhibitor 3-amino-1,2,4-triazole (3AT) abrogated DHC-induced expression of LC3-II, overexpression of the catalase gene increased expression of LC3-II protein, and knockdown decreased it. Additionally, DHC-induced autophagy was independent of p53 status. Collectively, DHC activates autophagy in a p53-independent manner and that may contribute to cytotoxicity of DHC.
Dihydrocapsaicin treatment depletes peptidergic nerve fibers of substance P and alters mast cell density in the respiratory tract of neonatal sheep.[Pubmed:10967206]
Regul Pept. 2000 Jul 28;91(1-3):97-106.
In the present study we administered Dihydrocapsaicin (DHC) to neonatal lambs to deplete C-fibers of neuropeptides. We measured the density of substance P (SP)-fibers in nasal septum to assess the effectiveness of the treatment at 3, 9, and 21 days. The numbers of mast cells in the upper and lower respiratory tract were determined at the same time points and histamine content was determined from lung tissue. DHC treatment depleted SP-fibers for up to the 21 day time point. This depletion was estimated as 85% in comparison with controls. In vehicle-treated lambs, the density of SP-fibers decreased progressively with age, but not to the degree of DHC-treated lambs whose SP-fibers were depleted from the initial 3-day measurement. In both, vehicle- and DHC-treated lambs, numbers of mast cells increased progressively with time; however, the density of mast cells was augmented in the entire respiratory tract of DHC-treated animals. Apparently, DHC treatment exerts a single and initial effect in increasing mast cells whereas time maintains a continuous influence; both factors exert their influence independently. Despite large numbers of mast cells in DHC-treated animals, histamine content in the lung had similar levels as controls. Our study provides fundamental data for a better understanding of conditions that may influence defense mechanisms dependent on the mast cell-nerve axis in the respiratory tract.
Effects of capsaicin, dihydrocapsaicin, and curcumin on copper-induced oxidation of human serum lipids.[Pubmed:16910741]
J Agric Food Chem. 2006 Aug 23;54(17):6436-9.
The oxidation of low-density lipoprotein (LDL) is believed to be the initiating factor for the development and progression of atherosclerosis. The active ingredients of spices such as chili and turmeric (capsaicin and curcumin, respectively) have been shown to reduce the susceptibility of LDL to oxidation. One of the techniques used to study the oxidation of LDL is to isolate LDL and subject it to metal-induced (copper or iron) oxidation. However, whole serum may represent a closer situation to in vivo conditions than using isolated LDL. We investigated the effects of different concentrations (0.1-3 microM) of capsaicin, Dihydrocapsaicin, and curcumin on copper-induced oxidation of serum lipoproteins. The lag time (before initiation of oxidation) and rate of oxidation (slope of propagation phase) were calculated. The lag time increased, and the rate of oxidation decreased with increasing concentrations of the tested antioxidants (p < 0.05). A 50% increase in lag time (from control) was observed at concentrations between 0.5 and 0.7 microM for capsaicin, Dihydrocapsaicin, and curcumin. This study shows that oxidation of serum lipids is reduced by capsaicinoids and curcumin in a concentration-dependent manner.
A validated HPLC-FLD method for analysis of intestinal absorption and metabolism of capsaicin and dihydrocapsaicin in the rat.[Pubmed:25462121]
J Pharm Biomed Anal. 2015 Jan 25;103:59-66.
A sensitive and selective reverse-phase high performance liquid chromatographic method with fluorescence detection has been developed for determination of capsaicin (8-methyl-N-vanillyl-(trans)-6-nonenamid) and Dihydrocapsaicin (8-methyl-N-vanillylnonanamide) in samples generated in rat small intestine luminal perfusion experiments. The experiments were designed to study the biotransformation of capsaicinoids in the small intestine in the rat. The chromatographic separation was performed at room temperature on a ZORBAX Eclipse((R)) XDB-C8 column using isocratic elution with a mobile phase consisting 0.05M orthophosphoric acid solution and acetonitrile (60:40, v/v; pH 3.0) with a flow rate of 1.5mL/min. Fluorescence detection was performed at excitation and emission wavelengths of 230 and 323nm, respectively. The method was evaluated for a number of validation characteristics (accuracy, repeatability and intermediate precision, limit of detection, limit of quantification and calibration range). The limit of detection (LOD) was 50ng/mL and the limit of quantification (LOQ) was 100ng/mL for both capsaicin and Dihydrocapsaicin reference standards dissolved in blank perfusate. The method was successfully applied for investigation of intestinal absorption of capsaicin and Dihydrocapsaicin while 30mug/mL standardized Capsicum extract - containing capsaicin and Dihydrocapsaicin - was luminally perfused for a 90min period. The structure of the glucuronide metabolites of capsaicin and Dihydrocapsaicin appeared in the perfusate was identified by mass spectrometry.


