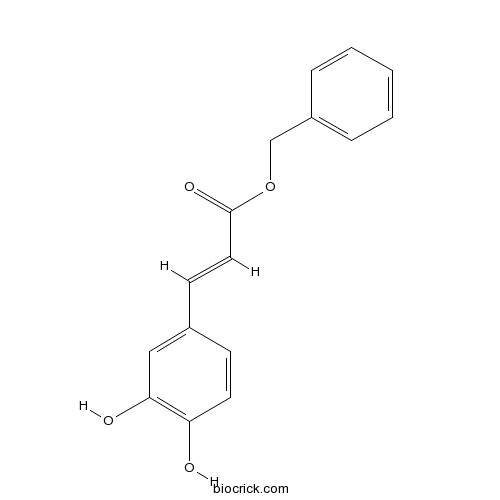
Benzyl CaffeateVEGFR(Flk-1/KDR) inhibitor CAS# 107843-77-6 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107843-77-6 | SDF | Download SDF |
| PubChem ID | 5919576 | Appearance | Powder |
| Formula | C16H14O4 | M.Wt | 270.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (E)-Benzyl 3-(3,4-Dihydroxyphenyl)Acrylate; Caffeic Acid Benzyl Ester;130734-47-3 | ||
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | benzyl (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | ||
| SMILES | C1=CC=C(C=C1)COC(=O)C=CC2=CC(=C(C=C2)O)O | ||
| Standard InChIKey | WWVKQTNONPWVEL-VQHVLOKHSA-N | ||
| Standard InChI | InChI=1S/C16H14O4/c17-14-8-6-12(10-15(14)18)7-9-16(19)20-11-13-4-2-1-3-5-13/h1-10,17-18H,11H2/b9-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzyl Caffeate Dilution Calculator

Benzyl Caffeate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6999 mL | 18.4993 mL | 36.9987 mL | 73.9973 mL | 92.4967 mL |
| 5 mM | 0.74 mL | 3.6999 mL | 7.3997 mL | 14.7995 mL | 18.4993 mL |
| 10 mM | 0.37 mL | 1.8499 mL | 3.6999 mL | 7.3997 mL | 9.2497 mL |
| 50 mM | 0.074 mL | 0.37 mL | 0.74 mL | 1.4799 mL | 1.8499 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.37 mL | 0.74 mL | 0.925 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ac-D-Ala-OH
Catalog No.:BCC3196
CAS No.:19436-52-3
- Curcumenol
Catalog No.:BCN3522
CAS No.:19431-84-6
- Selinidin
Catalog No.:BCN2711
CAS No.:19427-82-8
- N-Acetyl-5,6-dehydrololine
Catalog No.:BCN2007
CAS No.:194205-01-1
- Isohyenanchin
Catalog No.:BCN1187
CAS No.:19417-00-6
- Isosaponarin
Catalog No.:BCN2279
CAS No.:19416-87-6
- Rivulobirin B
Catalog No.:BCN1186
CAS No.:194145-29-4
- Dihydrocapsaicin
Catalog No.:BCN1017
CAS No.:19408-84-5
- 3-Amino-4-methylbenzamide
Catalog No.:BCC8614
CAS No.:19406-86-1
- 6,19-Dihydroxyurs-12-en-3-oxo-28-oic acid
Catalog No.:BCN1513
CAS No.:194027-11-7
- Cedrelopsin
Catalog No.:BCN7687
CAS No.:19397-28-5
- 10-O-Vanilloylaucubin
Catalog No.:BCN1185
CAS No.:193969-08-3
- CL-387785 (EKI-785)
Catalog No.:BCC6436
CAS No.:194423-06-8
- PD168393
Catalog No.:BCC1157
CAS No.:194423-15-9
- Taberpsychine
Catalog No.:BCN4047
CAS No.:19452-84-7
- 24-Norhopa-4(23),22(29)-diene-3β,6β-diol
Catalog No.:BCN1512
CAS No.:194613-74-6
- Rubrosterone
Catalog No.:BCN1511
CAS No.:19466-41-2
- GaTx2
Catalog No.:BCC6326
CAS No.:194665-85-5
- Melittoside
Catalog No.:BCN8413
CAS No.:19467-03-9
- Taxuspine X
Catalog No.:BCN6936
CAS No.:194782-02-0
- Dracoflavan B1
Catalog No.:BCN3589
CAS No.:194794-44-0
- Dracoflavan B2
Catalog No.:BCN3590
CAS No.:194794-47-3
- Dracoflavan C1
Catalog No.:BCN3587
CAS No.:194794-49-5
- Dracoflavan C2
Catalog No.:BCN3586
CAS No.:194794-50-8
Potential Protective Effects of Bioactive Constituents from Chinese Propolis against Acute Oxidative Stress Induced by Hydrogen Peroxide in Cardiac H9c2 Cells.[Pubmed:28337227]
Evid Based Complement Alternat Med. 2017;2017:7074147.
Chinese propolis (CP) is known as a health food but its beneficial effects in protecting cardiomyocytes remain elusive. Here, we investigated the effects of CP and its active compounds on hydrogen peroxide (H2O2) induced rats cardiomyocytes (H9c2) oxidative injury. Cell viability decreases induced by H2O2 were mitigated by different CP extracts using various solvents. From these active fractions, six active compounds were separated and identified. Among tested isolated compound, the cytoprotective activities of three caffeates, caffeic acid phenethyl ester (CAPE), Benzyl Caffeate (BZC), and cinnamyl caffeate (CNC), exerted stronger effects than chrysin, pinobanksin, and 3,4-dimethoxycinnamic acid (DMCA). These three caffeates also increased H9c2 cellular antioxidant potential, decreased intracellular calcium ion ([Ca(2+)]i) level, and prevented cell apoptosis. Overall, the cardiovascular protective effects of the CP might be attributed to its caffeates constituents (CAPE, BZC, and CNC) and provide evidence for its usage in complementary and alternative medicine.
Impact of Biohybrid Magnetite Nanoparticles and Moroccan Propolis on Adherence of Methicillin Resistant Strains of Staphylococcus aureus.[Pubmed:27618006]
Molecules. 2016 Sep 9;21(9). pii: molecules21091208.
Biofilm bacteria are more resistant to antibiotics than planktonic cells. Propolis possesses antimicrobial activity. Generally, nanoparticles containing heavy metals possess antimicrobial and antibiofilm properties. In this study, the ability of adherence of Methicillin Resistant Strains of Staphylococcus aureus (MRSA) to catheters treated with magnetite nanoparticles (MNPs), produced by three methods and functionalized with oleic acid and a hydro-alcoholic extract of propolis from Morocco, was evaluated. The chemical composition of propolis was established by gas chromatography mass spectrometry (GC-MS), and the fabricated nanostructures characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), Mossbauer spectroscopy and Fourrier transform infrared spectroscopy (FTIR). The capacity for impairing biofilm formation was dependent on the strain, as well as on the mode of production of MNPs. The co-precipitation method of MNPs fabrication using Fe(3+) and Na(2)SO(3) solution and functionalized with oleic acid and propolis was the most effective in the impairment of adherence of all MRSA strains to catheters (p < 0.001). The adherence of the strain MRSA16 was also significantly lower (p < 0.001) when the catheters were treated with the hybrid MNPs with oleic acid produced by a hydrothermal method. The anti-MRSA observed can be attributed to the presence of Benzyl Caffeate, pinocembrin, galangin, and isocupressic acid in propolis extract, along with MNPs. However, for MRSA16, the impairment of its adherence on catheters may only be attributed to the hybrid MNPs with oleic acid, since very small amount, if any at all of propolis compounds were added to the MNPs.
Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts.[Pubmed:26351514]
Evid Based Complement Alternat Med. 2015;2015:595393.
Propolis is a natural substance known to be beneficial for human health and used as a folk medicine in many parts of the world. In this study, phenolic profiles and antioxidant properties of Beijing propolis extracted by different ethanol/water solvents were analyzed. Our results reveal that phenolic compounds and antioxidant properties of propolis extracts were significantly dependent on the concentration of ethanol/water solvents. Totally, 29 phenolic compounds were identified: 12 phenolic acids, 13 flavonoids, and 4 phenolic acid esters. In particular, 75 wt.% ethanol/water solvent may be the best for the highest extraction yield and the strongest antioxidant properties. Caffeic acid, Benzyl Caffeate, phenethyl caffeate, 5-methoxy pinobanksin, pinobanksin, pinocembrin, pinobanksin-3-O-acetate, chrysin, and galangin were the characteristic compounds of Beijing propolis, and these compounds seem to verify that Beijing propolis may be poplar-type propolis. In addition, the presence of high level of pinobanksin-3-O-acetate in Chinese propolis may be a novel finding, representing one-third of all phenolics.
Inhibitory effects of caffeic acid ester analogues on free radicals and human liver microsome CYP1A2 activities.[Pubmed:21222608]
Med Chem. 2011 Mar;7(2):99-105.
Ethyl caffeate (EC), octyl caffeate(OC), Benzyl Caffeate(BC) and phenethyl caffeate(PC) were synthesized and evaluated for scavenging of superoxide anion, nitric oxide radical and 1,1-diphenyl-1-picrylhydrazyl radical (DPPH). Antioxidant activity was investigated with reducing power method. Pooled human liver microsome was used for investigating the effects on cytochrome P450 1A2 (CYP1A2) catalytic activities by using phenacetin as a substrate. Dixon and Cornish-Bowden plots were used for enzyme kinetic analysis. The EC, OC, BC and PC potentially inhibited superoxide anion, nitric oxide and DPPH radicals. IC(50) values of superoxide anion scavenging of EC, OC, BC and PC were 16.42, 79.83, 123.69 and 123.69 microg/ml, respectively. EC was more potent than OC and BC in terms of nitric oxide radical scavenger: IC(50) values of EC, OC and BC were 24.16, 37.34 and 52.64 microg/ml, respectively. In addition, the IC(50) values of EC, OC, BC and PC on DPPH radical scavenging were 70.00, 184.56, 285.34 and 866.54 microg/ ml, respectively. The IC(50) values of EC, OC, BC and PC on phenacetin O-deethylation were 124.98, 111.86, 156.68 and 31.05 microg/ml, respectively. Enzyme kinetics showed that the type of inhibition mechanism was mixed-type. The result of this study shows that caffeic acid ester analogues potentially scavenge free radicals and inhibit catalytic activity of CYP1A2. This may lead to important implications in the prevention of CYP1A2-mediated chemical carcinogenesis.
Simultaneous quantification of eight major bioactive phenolic compounds in Chinese propolis by high-performance liquid chromatography.[Pubmed:19634328]
Nat Prod Commun. 2009 Jun;4(6):813-8.
A simple, sensitive and specific high-performance liquid chromatography-UV (HPLC-UV) method has been developed to simultaneously quantify the eight major bioactive phenolic compounds in Chinese propolis, namely caffeic acid, isoferulic acid, 3,4-dimethoxycinnamic acid, pinobanksin 5-methyl ether, pinocembrin, Benzyl Caffeate, chrysin and galangin. This HPLC assay was performed on an Agilent Zorbax Extend-C18 (250 x 4.6 mm, 5 microm) column with a gradient of methanol and 0.2% aqueous acetic acid (v/v) in 50 min, at a flow rate of 1.0 mL/min, and detected at 290 nm. All calibration curves showed good linearity (r2 > 0.999) within the test ranges. The intra- and inter-day assay precision (RSD) of eight phenolic compounds were in the range of 0.07-4.92%. The recoveries were between 98.3% and 104.8%. This assay was applied to the evaluation of nineteen samples from different origins in China. The results indicated that the developed assay could be readily utilized for the quality control of propolis.
Selective analysis of phenolic compounds in propolis by HPLC-MS/MS.[Pubmed:17654520]
Phytochem Anal. 2008 Jan-Feb;19(1):32-9.
Phenolic compounds (flavonoids and phenolic acid derivatives) are major active constituents of the resinous fraction of propolis, and also represent its allergenic principles. We have developed a chromatography electrospray ionisation tandem mass spectrometry (HPLC-ESI-MS/MS) method to characterise the polyphenolic fraction of propolis rapidly and quali-quantitatively. With precursor ion scanning, selective detection of caffeic esters was easily achieved, confirming the identification of prenyl caffeate, Benzyl Caffeate and phenylethyl caffeate by comparison with synthetic standards. The ionisation and fragmentation behaviour of the major propolis flavonoids was rationalised and applied to selected real samples. Taken together, the results of this study show that the introduction of precursor ion analysis leads to a significant improvement in the characterisation of the phenolic fraction of propolis, paving the way to the establishment of a better quality control for this important natural remedy.
Evaluation of the main contact allergens in propolis (1995 to 2005).[Pubmed:16242084]
Dermatitis. 2005 Sep;16(3):127-9.
BACKGROUND: Propolis, the bee glue, is increasingly used in biocosmetics and for the self-treatment of various diseases. OBJECTIVE: Patients reacting to propolis were requested to participate in further testing with the breakdown constituents of the bee glue. METHODS: Twenty-seven patients agreed to be tested with 18 constituents, including four caffeates (the typical allergens of propolis) derived from the sticky exudates of poplar buds. RESULTS: Seven patients did not react to the propolis constituents tested. In the remaining 20 patients, the four caffeates produced strong reactions. Phenylethyl caffeate, which produced positive reactions in 20 patients, was the leading contact allergen. Benzyl Caffeate elicited strong responses in 18 patients, and 3-methyl-2-butenyl caffeate produced reactions in 17 patients. Geranyl caffeate produced positive reactions in 11 patients. The flavonoid tectochrysin gave positive results in 2 patients; ferulic acid, coumaric acid, and methyl cinnamate produced weak responses. CONCLUSIONS: In middle Europe, the caffeates are the responsible allergens in propolis allergy. Patients from other countries, where poplar trees do not grow, become allergic to other propolis constituents but not to the caffeates.
Caffeic acid phenethyl ester (CAPE) analogues: potent nitric oxide inhibitors from the Netherlands propolis.[Pubmed:12673030]
Biol Pharm Bull. 2003 Apr;26(4):487-91.
The MeOH and water extracts of the Netherlands propolis were tested for their inhibitory activity toward nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophage-like J774.1 cells. Both of the extract possessed significant NO inhibitory activity with IC(50) values of 23.8 and 51.5 microg/ml, respectively. Then 13 phenolic compounds obtained from the MeOH extract showing stronger NO inhibition were examined on their NO inhibitory activities. Caffeic acid phenethyl ester (CAPE) analogues, i.e., Benzyl Caffeate, CAPE and cinnamyl caffeate, possessed most potent NO inhibitory activities with IC(50) values of 13.8, 7.64 and 9.53 microM, respectively, which were two- to four-fold stronger than the positive control N(G)-monomethyl-L-arginine (L-NMMA; IC(50), 32.9 microM). Further study on the synthetic analogues of CAPE revealed that both of 3-phenylpropyl caffeate (18; IC(50), 7.34 microM) and 4-phenylbutyl caffeate (19; IC(50), 6.77 microM) possessed stronger NO inhibitory activity than CAPE (10) and that elongation of alkyl side chain of alcoholic parts of caffeic acid esters enhance the NO inhibitory activity. In addition, it was found that CAPE analogues having longer carbon chain (>C(5)) in alcoholic part showed toxic effects toward J774.1 cells. This NO inhibitory effect may directly correlate with antiinflammatory properties of the Netherlands propolis.
Constituents of Chinese propolis and their antiproliferative activities.[Pubmed:12027739]
J Nat Prod. 2002 May;65(5):673-6.
Two new flavonoids, 3-O-[(S)-2-methylbutyroyl]pinobanksin (1) and 6-cinnamylchrysin (2), were isolated from the EtOAc-soluble fraction of the MeOH extract of Chinese propolis, along with 12 known compounds (3-14). The structures of the isolated compounds were elucidated on the basis of spectroscopic and chemical analyses. The isolated compounds were tested for their antiproliferative activity toward five different cancer cell lines. Benzyl Caffeate (13) and phenethyl caffeate (14) showed potent antiproliferative activity toward tested cell lines with a selective activity toward colon 26-L5 carcinoma cell line (EC(50) values: 13, 1.01; 14, 0.30 microM).
Chemical composition and antimicrobial activity of European propolis.[Pubmed:10739103]
Z Naturforsch C. 2000 Jan-Feb;55(1-2):70-5.
Three propolis samples from Austria, Germany and France were investigated by GC/MS, where eleven compounds were being new for propolis. The samples showed some similarities in their qualitative composition. Phenylethyl-trans-caffeate, benzyl ferulate and galangin were predominant in German propolis. Benzyl Caffeate was predominant in French sample. Pinocembrin was predominant in French and Austrian propolis and trans-p-coumaric acid was predominant in all samples. The antimicrobial activity against Staphylococcus aureus; Escherichia coli, and Candida albicans was evaluated. German propolis showed the highest antimicrobial activity against Staphylococcus aureus and Escherichia coli. While Austrian propolis has the highest activity against Candida albicans. French propolis was effective against all pathogens but less than German and Austrian propolis.
[Isolation and identification of a new cinnamate ester from liaoxi propolis].[Pubmed:9772700]
Yao Xue Xue Bao. 1996;31(7):558-60.
A new cinnamate ester drivitive (II) and three flavonoids (I, III, IV) were isolated from Liaoxi propolis. Their chemical structures were established as Benzyl Caffeate (II), 7-O-methylchrysin (I), genkwanin (III) and rhamnazin (IV) by spectral analysis. II is a new natural compound; I, III and IV were found from the propolis for the first time.


