SB 242235MAPK inhibitor CAS# 193746-75-7 |
- Skepinone-L
Catalog No.:BCC1953
CAS No.:1221485-83-1
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 193746-75-7 | SDF | Download SDF |
| PubChem ID | 9863367 | Appearance | Powder |
| Formula | C19H20FN5O | M.Wt | 353.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 48 mg/mL (135.83 mM) *"≥" means soluble, but saturation unknown. | ||
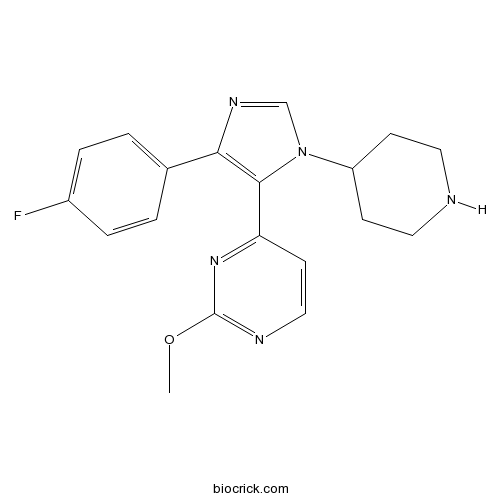
| Chemical Name | 4-[5-(4-fluorophenyl)-3-piperidin-4-ylimidazol-4-yl]-2-methoxypyrimidine | ||
| SMILES | COC1=NC=CC(=N1)C2=C(N=CN2C3CCNCC3)C4=CC=C(C=C4)F | ||
| Standard InChIKey | PDTYLGXVBIWRIM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H20FN5O/c1-26-19-22-11-8-16(24-19)18-17(13-2-4-14(20)5-3-13)23-12-25(18)15-6-9-21-10-7-15/h2-5,8,11-12,15,21H,6-7,9-10H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | SB-242235 is a potent and selective p38 MAP kinase inhibitor with IC50 of 1.0 uM.
IC50 Value: 1.0 uM [1]
Target: p38 MAPK
in vitro: SB 242235 inhibited intracellular p38 activity, human chondrocytes were treated with different doses of SB 242235 prior to stimulation with IL-1_ for 15 min. MAPKAP K2 was then isolated from these cells and assayed using HSP27 as a substrate. SB 242235 dose-dependently inhibited the activation of MAPKAP K2 with an IC50 of 1.0 uM [1].
in vivo: SB-242235 demonstrates generally favourable pharmacokinetic properties in all species examined(including rat, dog and monkey). Systemic plasma clearance was high in rat, but in the non-rodent species SB-242235 demonstrated low to moderate clearance with plasma half-lives > 4h. Oral bioavailability in each preclinical species was high. In rat and monkey, SB-242235 demonstrated non-linear elimination kinetics that manifested as a decrease in clearance with increasing dose and apparent oral bioavailability > 100% at high oral doses [2].In the skin of SKH-1 hairless mice, SB242235, prior to UVB irradiation, blocked activation of the p38 MAPK cascade, and abolished MAPKAPK-2 kinase activity and phosphorylation of HSP27. Moreover, SB242235 inhibited expression of the pro-inflammatory cytokines interleukin (IL)-6 and KC (murine IL-8) and COX-2 [3]. The preclinical pharmacokinetics of SB-242235 have been described previously. The present studies were conducted to describe the in vitro metabolic rates and routes of SB-242235 metabolism, to characterize its in vivo preclinical metabolism, and to use these data to aid in the prediction of the pharmacokinetic behaviour of SB-242235 in man [4]. References: | |||||

SB 242235 Dilution Calculator

SB 242235 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8297 mL | 14.1487 mL | 28.2973 mL | 56.5947 mL | 70.7434 mL |
| 5 mM | 0.5659 mL | 2.8297 mL | 5.6595 mL | 11.3189 mL | 14.1487 mL |
| 10 mM | 0.283 mL | 1.4149 mL | 2.8297 mL | 5.6595 mL | 7.0743 mL |
| 50 mM | 0.0566 mL | 0.283 mL | 0.5659 mL | 1.1319 mL | 1.4149 mL |
| 100 mM | 0.0283 mL | 0.1415 mL | 0.283 mL | 0.5659 mL | 0.7074 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SB 242235 is a selective inhibitor of p38 mitogen activated protein kinase (MAPK) with IC50 values of 0.019μM and 1μM for p38α MAPK and p38β MAPK, respectively [1].
SB 242235 is developed for the treatment of cytokine-mediated diseases such as rheumatoid arthritis (RA). It belongs to the pyridinyl imidazole class and shows potent anti-inflammatory activity without affecting 5-lipoxygenase or cyclooxygenase-1. SB 242235 is found to be a selective p38MAPK inhibitor. It has no significant inhibitory activity against ERK and JNK. In normal rats, administration of SB 242235 significantly inhibits the production of TNFα induced by LPS. When treated with AIA rats, SB 242235 inhibits paw edema with ED50 value of about 30 mg/kg. It also remarkably suppresses the joint damage of the rats. Moreover, SB 242235 exerts a significant inhibition of the cytokine IL-6 at dose of 60 mg/kg [2].
References:
[1] Ward K W, Proksch J W, Salyers K L, et al. SB-242235, a selective inhibitor of p38 mitogenactivated protein kinase. I: Preclinical pharmacokinetics. xenobiotica, 2002, 32(3): 221-233.
[2] Badger A M, Griswold D E, Kapadia R, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis & Rheumatism, 2000, 43(1): 175-183.
- Aminoguanidine hydrochloride
Catalog No.:BCC6795
CAS No.:1937-19-5
- 8beta-Methoxyatractylenolide I
Catalog No.:BCN7594
CAS No.:193694-24-5
- Methyl 4-hydroxycinnamate
Catalog No.:BCN4014
CAS No.:19367-38-5
- TAS 301
Catalog No.:BCC6214
CAS No.:193620-69-8
- BRL-15572
Catalog No.:BCC5065
CAS No.:193611-72-2
- SB 216641 hydrochloride
Catalog No.:BCC6987
CAS No.:193611-67-5
- Terrestrosin K
Catalog No.:BCN2935
CAS No.:193605-07-1
- GB 1b
Catalog No.:BCN7385
CAS No.:19360-72-6
- Calcifediol
Catalog No.:BCC4949
CAS No.:19356-17-3
- SB 239063
Catalog No.:BCC1923
CAS No.:193551-21-2
- IPAG
Catalog No.:BCC5662
CAS No.:193527-91-2
- Mulberroside F
Catalog No.:BCN2908
CAS No.:193483-95-3
- Cortistatin 14
Catalog No.:BCC6010
CAS No.:193829-96-8
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Fmoc-β-Homo-Leu-OH
Catalog No.:BCC2631
CAS No.:193887-44-4
- 3-Phenyl-1,2-dihydroacenaphthylene-1,2-diol
Catalog No.:BCN7178
CAS No.:193892-33-0
- SC 19220
Catalog No.:BCC6968
CAS No.:19395-87-0
- Fmoc-ß-HoAla-OH
Catalog No.:BCC3225
CAS No.:193954-26-6
- Fmoc- ß-HoIle-OH
Catalog No.:BCC3237
CAS No.:193954-27-7
- Fmoc-β-HoPhe-OH
Catalog No.:BCC3241
CAS No.:193954-28-8
- 10-O-Vanilloylaucubin
Catalog No.:BCN1185
CAS No.:193969-08-3
- Cedrelopsin
Catalog No.:BCN7687
CAS No.:19397-28-5
- 6,19-Dihydroxyurs-12-en-3-oxo-28-oic acid
Catalog No.:BCN1513
CAS No.:194027-11-7
- 3-Amino-4-methylbenzamide
Catalog No.:BCC8614
CAS No.:19406-86-1
Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis.[Pubmed:10643714]
Arthritis Rheum. 2000 Jan;43(1):175-83.
OBJECTIVE: To evaluate the effects of SB 242235, a potent and selective inhibitor of p38 mitogen-activated protein (MAP) kinase, on joint integrity in rats with adjuvant-induced arthritis (AIA). METHODS: Male Lewis rats with AIA were orally treated either prophylactically (days 0-20) or therapeutically (days 10-20) with SB 242235. Efficacy was determined by measurements of paw inflammation, dual-energy x-ray absorptiometry for bone-mineral density (BMD), magnetic resonance imaging (MRI), microcomputed tomography (CT), and histologic evaluation. Serum tumor necrosis factor alpha (TNFalpha) in normal (non-AIA) rats and serum interleukin-6 (IL-6) levels in rats with AIA were measured as markers of the antiinflammatory effects of the compound. RESULTS: SB 242235 inhibited lipopolysaccharide-stimulated serum levels of TNFalpha in normal rats, with a median effective dose of 3.99 mg/kg. When SB 242235 was administered to AIA rats prophylactically on days 0-20, it inhibited paw edema at 30 mg/kg and 10 mg/kg per day by 56% and 33%, respectively. Therapeutic administration on days 10-20 was also effective, and inhibition of paw edema was observed at 60, 30, and 10 mg/kg (73%, 51%, and 19%, respectively). Significant improvement in joint integrity was demonstrated by showing normalization of BMD and also by MRI and micro-CT analysis. Protection of bone, cartilage, and soft tissues was also shown histologically. Serum IL-6 levels were decreased in AIA rats treated with the 60 mg/kg dose of compound. CONCLUSION: Symptoms of AIA in rats were significantly reduced by both prophylactic and therapeutic treatment with the p38 MAP kinase inhibitor, SB 242235. Results from measurements of paw inflammation, assessment of BMD, MRI, and micro-CT indicate that this compound exerts a protective effect on joint integrity, and thus appears to have disease-modifying properties.
SB-242235, a selective inhibitor of p38 mitogen-activated protein kinase. I: preclinical pharmacokinetics.[Pubmed:11958561]
Xenobiotica. 2002 Mar;32(3):221-33.
1. SB-242235 (1-(4-piperidinyl)-4-(4-fluorophenyl)-5-(2-methoxy-4-pyrimidinyl) imidazole) is a potent and selective p38 MAP kinase inhibitor that may be an effective therapy for cytokine-mediated diseases such as autoimmune or inflammatory diseases. The present studies were conducted to evaluate the pharmacokinetics of SB-242235 in several preclinical species, including rat, dog and monkey. 2. SB-242235 demonstrates generally favourable pharmacokinetic properties in all species examined. Systemic plasma clearance was high in rat, but in the non-rodent species SB-242235 demonstrated low to moderate clearance with plasma half-lives > 4h. Oral bioavailability in each preclinical species was high. In rat and monkey, SB-242235 demonstrated non-linear elimination kinetics that manifested as a decrease in clearance with increasing dose and apparent oral bioavailability > 100% at high oral doses. Furthermore, SB-242235 displayed concentration-dependent plasma protein binding over a concentration range of 1000-10,000 ng ml(-1). 3. In conclusion, SB-242235 demonstrates high oral bioavailability across the major preclinical species, and may thus be a useful tool compound for investigation of the role of p38 inhibition in various disease states. However, the observations of non-linear protein binding and disposition also suggest the need for caution in the design of and data interpretation from such studies.
Differential effects of SB 242235, a selective p38 mitogen-activated protein kinase inhibitor, on IL-1 treated bovine and human cartilage/chondrocyte cultures.[Pubmed:11069728]
Osteoarthritis Cartilage. 2000 Nov;8(6):434-43.
The p38 MAP kinase inhibitor, SB 242235, was evaluated for its effects on the metabolism of bovine and human cartilage and primary chondrocyte cultures. SB 242235 had no effect on proteoglycan synthesis (PG) in bovine articular cartilage explants (BAC), as measured by [(35)S]-sulfate incorporation into glycosaminoglycans (GAGs). In addition, the compound had no effect on IL-1 alpha-induced GAG release from these cultures. However, there was a potent, dose-dependent inhibition of nitric oxide (NO) release from IL-1 alpha-stimulated BAC with an IC(50)of approximately 0.6 microM, with similar effects observed in primary chondrocytes. The effect on BAC was time dependent, and mechanistically did not appear to be the result of inhibition of protein kinase C (PKC), protein kinase A (PKA) or MEK-1. The effect on NO release in bovine chondrocytes was at the level of inducible nitric oxide synthase (iNOS) gene expression, which was inhibited at similar concentrations as nitrite production. In primary human chondrocytes, IL-1 beta induction of p38 MAP kinase was inhibited by SB 242235 with an IC(50)of approximately 1 microM. Surprisingly, however, treatment of IL-beta-stimulated human cartilage or chondrocytes with SB 242235 did not inhibit either NO production or the induction of iNOS. On the other hand, the natural product hymenialdisine (HYM), a protein tyrosine kinase (PTK) inhibitor, inhibited NO production and iNOS in both species. In contrast to the differential control of iNOS, PGE(2)was inhibited by SB 242235 in both IL-1-stimulated bovine and human chondrocyte cultures. These studies indicate that there are species differences in the control of iNOS by p38 inhibitors and also that different pathways may control IL-1-induced proteoglycan breakdown and NO production.
SB-242235, a selective inhibitor of p38 mitogen-activated protein kinase. II: in vitro and in vivo metabolism studies and pharmacokinetic extrapolation to man.[Pubmed:11958562]
Xenobiotica. 2002 Mar;32(3):235-50.
1. Inhibition of p38 MAP kinase has been investigated extensively as a potential therapy for cytokine-mediated diseases such as autoimmune and inflammatory diseases. SB-242235 (1-(4-piperidinyl)-4-(4-fluorophenyl)-5-(2-methoxy-4-pyrimidinyl) imidazole) is a potent and selective p38 MAP kinase inhibitor; the preclinical pharmacokinetics of SB-242235 have been described previously. The present studies were conducted to describe the in vitro metabolic rates and routes of SB-242235 metabolism, to characterize its in vivo preclinical metabolism, and to use these data to aid in the prediction of the pharmacokinetic behaviour of SB-242235 in man. 2. SB-242235 was metabolically stable in rat, dog, monkey and human hepatic microsomes, isolated hepatocytes and liver slices in vitro. The in vivo preclinical metabolism studies were consistent with the in vitro findings; SB-242235 was minimally metabolized, and was primarily excreted unchanged in the urine (45 and 67% of the administered dose in the rat and monkey, respectively). 3. Allometric scaling using various correction factors predicted that SB-242235 would have low clearance in man with a predicted half-life ranging from 11.5 to 18.7h. This prediction was consistent with the observed mean half-life of 16.4h in the first-in-man study for SB-242235. An allometric scaling method with a correction for interspecies differences in glomerular filtration rate provided the most accurate prediction of the pharmacokinetic behaviour of SB-242235 in humans, although the clinical data also highlight potential difficulties in conducting prospective allometry.


