LY2228820P38 MAP kinase inhibitor CAS# 862507-23-1 |
- SB 203580
Catalog No.:BCC3663
CAS No.:152121-47-6
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 862507-23-1 | SDF | Download SDF |
| PubChem ID | 11570805 | Appearance | Powder |
| Formula | C26H37FN6O6S2 | M.Wt | 612.74 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ralimetinib dimesylate; LY2228820 dimesylate; LY2228820 2MsOH; | ||
| Solubility | DMSO : 61 mg/mL (99.55 mM; Need ultrasonic and warming) | ||
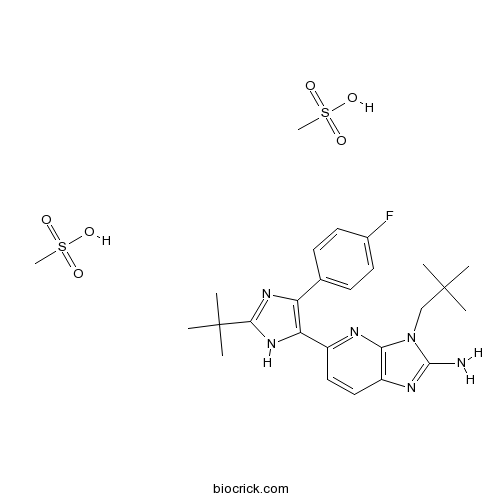
| Chemical Name | 5-[2-tert-butyl-4-(4-fluorophenyl)-1H-imidazol-5-yl]-3-(2,2-dimethylpropyl)imidazo[4,5-b]pyridin-2-amine;methanesulfonic acid | ||
| SMILES | CC(C)(C)CN1C2=C(C=CC(=N2)C3=C(N=C(N3)C(C)(C)C)C4=CC=C(C=C4)F)N=C1N.CS(=O)(=O)O.CS(=O)(=O)O | ||
| Standard InChIKey | NARMJPIBAXVUIE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H29FN6.2CH4O3S/c1-23(2,3)13-31-20-17(28-22(31)26)12-11-16(27-20)19-18(14-7-9-15(25)10-8-14)29-21(30-19)24(4,5)6;2*1-5(2,3)4/h7-12H,13H2,1-6H3,(H2,26,28)(H,29,30);2*1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LY2228820 is a novel and potent inhibitor of p38 MAPK with IC50 of 7 nM. | |||||
| Targets | p38α | |||||
| IC50 | 7 nM | |||||

LY2228820 Dilution Calculator

LY2228820 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.632 mL | 8.1601 mL | 16.3201 mL | 32.6403 mL | 40.8003 mL |
| 5 mM | 0.3264 mL | 1.632 mL | 3.264 mL | 6.5281 mL | 8.1601 mL |
| 10 mM | 0.1632 mL | 0.816 mL | 1.632 mL | 3.264 mL | 4.08 mL |
| 50 mM | 0.0326 mL | 0.1632 mL | 0.3264 mL | 0.6528 mL | 0.816 mL |
| 100 mM | 0.0163 mL | 0.0816 mL | 0.1632 mL | 0.3264 mL | 0.408 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2228820 is a potent, selective, ATP-competitive small-molecule inhibitor inhibiting α- and β-isoforms of p38 MAPK with IC(50) values of 5.3nM and 3.2 nM, respectively1.
In multiple myeloma (MM) cell lines, LY2228820 enhanced the cytotoxicity of bortezomiba via reducing bortezomib-induced phosphorylation of heat shock protein 27 (HSP27). LY2228820 significantly reduced IL-6 secretion from BM mononuclear cells (BMMNCs) and long term cultured-BM stromal cells (LT-BMSCs). Besides, LY2228820 can also inhibit secretion of macrophage inflammatory protein-1a (MIP-1a) in BMMNCs, CD138+ patient MM cells and normal CD14+ osteoclast cells 2.
Studies in mice implanted with B16-F10 melanoma showed that orally administered LY2228820 can effectively suppress the tumor-phospho-MK2 expression. Treatment of LY2228820 caused a significant tumor growth delay in A549 NSCLC xenograft models1.
References:
1. Campbell RM1, Anderson BD, Brooks NA, Brooks HB, Chan EM, De Dios A, Gilmour R, Graff JR, Jambrina E, Mader M, McCann D, Na S, Parsons SH, Pratt SE, Shih C, Stancato LF, Starling JJ, Tate C, Velasco JA, Wang Y, Ye XS.Characterization of LY2228820 dimesylate, a potent and selective inhibitor of p38 MAPK with antitumor activity. Mol Cancer Ther. 2014 Feb;13(2):364-74.
2. Ishitsuka K1, Hideshima T, Neri P, Vallet S, Shiraishi N, Okawa Y, Shen Z, Raje N, Kiziltepe T, Ocio EM, Chauhan D, Tassone P, Munshi N, Campbell RM, Dios AD, Shih C, Starling JJ, Tamura K, Anderson KC. p38 mitogen-activated protein kinase inhibitor LY2228820 enhances bortezomib-induced cytotoxicity and inhibits osteoclastogenesis in multiple myeloma; therapeutic implications. Br J Haematol. 2008 May;141(5):598-606.
- Imatinib hydrochloride
Catalog No.:BCC1644
CAS No.:862366-25-4
- RA VII
Catalog No.:BCN3512
CAS No.:86229-97-2
- Valeriotriate B
Catalog No.:BCN6751
CAS No.:862255-64-9
- Mirodenafil
Catalog No.:BCC5254
CAS No.:862189-95-5
- Nandrolone undecylate
Catalog No.:BCC9090
CAS No.:862-89-5
- Anamorelin hydrochloride
Catalog No.:BCC1364
CAS No.:861998-00-7
- 7-Methoxy-3,4-dihydro-1-naphthalenylacetonitrile
Catalog No.:BCC8781
CAS No.:861960-34-1
- 2''-O-Beta-L-Galorientin
Catalog No.:BCN3804
CAS No.:861691-37-4
- A-740003
Catalog No.:BCC1322
CAS No.:861393-28-4
- SKF 86466 hydrochloride
Catalog No.:BCC7795
CAS No.:86129-54-6
- Fmoc-D-Trp-OH
Catalog No.:BCC3559
CAS No.:86123-11-7
- Fmoc-D-Phe-OH
Catalog No.:BCC3537
CAS No.:86123-10-6
- IKK-3 Inhibitor
Catalog No.:BCC1643
CAS No.:862812-98-4
- Salvianan
Catalog No.:BCN3545
CAS No.:862832-46-0
- Ganoderic acid TR
Catalog No.:BCN3207
CAS No.:862893-75-2
- Isoiridogermanal
Catalog No.:BCN7613
CAS No.:86293-25-6
- 10-Hydroxycanthin-6-one
Catalog No.:BCN3906
CAS No.:86293-41-6
- alpha-Amyrin acetate
Catalog No.:BCN4410
CAS No.:863-76-3
- Azilsartan Medoxomil
Catalog No.:BCC5021
CAS No.:863031-21-4
- Azilsartan medoxomil monopotassium
Catalog No.:BCC4089
CAS No.:863031-24-7
- Impurity C of Calcitriol
Catalog No.:BCC5384
CAS No.:86307-44-0
- Dasatinib monohydrate
Catalog No.:BCN2177
CAS No.:863127-77-9
- 8-epi-Chlorajapolide F
Catalog No.:BCN6426
CAS No.:863301-69-3
- PSI-6206
Catalog No.:BCC3609
CAS No.:863329-66-2
A First-in-Human Phase I Study of the Oral p38 MAPK Inhibitor, Ralimetinib (LY2228820 Dimesylate), in Patients with Advanced Cancer.[Pubmed:26581242]
Clin Cancer Res. 2016 Mar 1;22(5):1095-102.
PURPOSE: p38 MAPK regulates the production of cytokines in the tumor microenvironment and enables cancer cells to survive despite oncogenic stress, radiotherapy, chemotherapy, and targeted therapies. Ralimetinib (LY2228820 dimesylate) is a selective small-molecule inhibitor of p38 MAPK. This phase I study aimed to evaluate the safety and tolerability of ralimetinib, as a single agent and in combination with tamoxifen, when administered orally to patients with advanced cancer. EXPERIMENTAL DESIGN: The study design consisted of a dose-escalation phase performed in a 3+3 design (Part A; n = 54), two dose-confirmation phases [Part B at 420 mg (n = 18) and Part C at 300 mg (n = 8)], and a tumor-specific expansion phase in combination with tamoxifen for women with hormone receptor-positive metastatic breast cancer refractory to aromatase inhibitors (Part D; n = 9). Ralimetinib was administered orally every 12 hours on days 1 to 14 of a 28-day cycle. RESULTS: Eighty-nine patients received ralimetinib at 11 dose levels (10, 20, 40, 65, 90, 120, 160, 200, 300, 420, and 560 mg). Plasma exposure of ralimetinib (Cmax and AUC) increased in a dose-dependent manner. After a single dose, ralimetinib inhibited p38 MAPK-induced phosphorylation of MAPKAP-K2 in peripheral blood mononuclear cells. The most common adverse events, possibly drug-related, included rash, fatigue, nausea, constipation, pruritus, and vomiting. The recommended phase II dose was 300 mg every 12 hours as monotherapy or in combination with tamoxifen. Although no patients achieved a complete response or partial response,19 patients (21.3%) achieved stable disease with a median duration of 3.7 months, with 9 of these patients on study for >/= 6 cycles. CONCLUSIONS: Ralimetinib demonstrated acceptable safety, tolerability, and pharmacokinetics for patients with advanced cancer.
Characterization of LY2228820 dimesylate, a potent and selective inhibitor of p38 MAPK with antitumor activity.[Pubmed:24356814]
Mol Cancer Ther. 2014 Feb;13(2):364-74.
p38alpha mitogen-activated protein kinase (MAPK) is activated in cancer cells in response to environmental factors, oncogenic stress, radiation, and chemotherapy. p38alpha MAPK phosphorylates a number of substrates, including MAPKAP-K2 (MK2), and regulates the production of cytokines in the tumor microenvironment, such as TNF-alpha, interleukin-1beta (IL-1beta), IL-6, and CXCL8 (IL-8). p38alpha MAPK is highly expressed in human cancers and may play a role in tumor growth, invasion, metastasis, and drug resistance. LY2228820 dimesylate (hereafter LY2228820), a trisubstituted imidazole derivative, is a potent and selective, ATP-competitive inhibitor of the alpha- and beta-isoforms of p38 MAPK in vitro (IC(50) = 5.3 and 3.2 nmol/L, respectively). In cell-based assays, LY2228820 potently and selectively inhibited phosphorylation of MK2 (Thr334) in anisomycin-stimulated HeLa cells (at 9.8 nmol/L by Western blot analysis) and anisomycin-induced mouse RAW264.7 macrophages (IC(50) = 35.3 nmol/L) with no changes in phosphorylation of p38alpha MAPK, JNK, ERK1/2, c-Jun, ATF2, or c-Myc LY2228820 also reduced TNF-alpha secretion by lipopolysaccharide/IFN-gamma-stimulated macrophages (IC(50) = 6.3 nmol/L). In mice transplanted with B16-F10 melanoma, tumor phospho-MK2 (p-MK2) was inhibited by LY2228820 in a dose-dependent manner [threshold effective dose (TED)(70) = 11.2 mg/kg]. Significant target inhibition (>40% reduction in p-MK2) was maintained for 4 to 8 hours following a single 10 mg/kg oral dose. LY2228820 produced significant tumor growth delay in multiple in vivo cancer models (melanoma, non-small cell lung cancer, ovarian, glioma, myeloma, breast). In summary, LY2228820 is a p38 MAPK inhibitor, which has been optimized for potency, selectivity, drug-like properties (such as oral bioavailability), and efficacy in animal models of human cancer.
LY2228820 dimesylate, a selective inhibitor of p38 mitogen-activated protein kinase, reduces angiogenic endothelial cord formation in vitro and in vivo.[Pubmed:23335506]
J Biol Chem. 2013 Mar 1;288(9):6743-53.
LY2228820 dimesylate is a highly selective small molecule inhibitor of p38alpha and p38beta mitogen-activated protein kinases (MAPKs) that is currently under clinical investigation for human malignancies. p38 MAPK is implicated in a wide range of biological processes, in particular those that support tumorigenesis. One such process, angiogenesis, is required for tumor growth and metastasis, and many new cancer therapies are therefore directed against the tumor vasculature. Using an in vitro co-culture endothelial cord formation assay, a surrogate of angiogenesis, we investigated the role of p38 MAPK in growth factor- and tumor-driven angiogenesis using LY2228820 dimesylate treatment and by shRNA gene knockdown. p38 MAPK was activated in endothelial cells upon growth factor stimulation, with inhibition by LY2228820 dimesylate treatment causing a significant decrease in VEGF-, bFGF-, EGF-, and IL-6-induced endothelial cord formation and an even more dramatic decrease in tumor-driven cord formation. In addition to involvement in downstream cytokine signaling, p38 MAPK was important for VEGF, bFGF, EGF, IL-6, and other proangiogenic cytokine secretion in stromal and tumor cells. LY2228820 dimesylate results were substantiated using p38alpha MAPK-specific shRNA and shRNA against the downstream p38 MAPK effectors MAPKAPK-2 and HSP27. Using in vivo models of functional neoangiogenesis, LY2228820 dimesylate treatment reduced hemoglobin content in a plug assay and decreased VEGF-A-stimulated vascularization in a mouse ear model. Thus, p38alpha MAPK is implicated in tumor angiogenesis through direct tumoral effects and through reduction of proangiogenic cytokine secretion via the microenvironment.


