Ganoderic acid TRCAS# 862893-75-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 862893-75-2 | SDF | Download SDF |
| PubChem ID | 102004381 | Appearance | Powder |
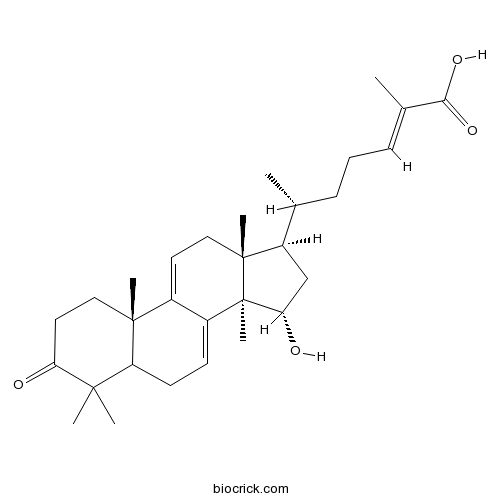
| Formula | C30H44O4 | M.Wt | 468.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E,6R)-6-[(10S,13R,14R,15S,17R)-15-hydroxy-4,4,10,13,14-pentamethyl-3-oxo-1,2,5,6,12,15,16,17-octahydrocyclopenta[a]phenanthren-17-yl]-2-methylhept-2-enoic acid | ||
| SMILES | CC(CCC=C(C)C(=O)O)C1CC(C2(C1(CC=C3C2=CCC4C3(CCC(=O)C4(C)C)C)C)C)O | ||
| Standard InChIKey | SNZQBBATMLGADX-UXRQJHJJSA-N | ||
| Standard InChI | InChI=1S/C30H44O4/c1-18(9-8-10-19(2)26(33)34)22-17-25(32)30(7)21-11-12-23-27(3,4)24(31)14-15-28(23,5)20(21)13-16-29(22,30)6/h10-11,13,18,22-23,25,32H,8-9,12,14-17H2,1-7H3,(H,33,34)/b19-10+/t18-,22-,23?,25+,28-,29-,30-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Ganoderic acid TR, a new lanostanoid with 5α-reductase inhibitory activity from the fruiting body of Ganoderma lucidum. |
| Targets | 5-alpha Reductase |

Ganoderic acid TR Dilution Calculator

Ganoderic acid TR Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1336 mL | 10.6678 mL | 21.3356 mL | 42.6712 mL | 53.339 mL |
| 5 mM | 0.4267 mL | 2.1336 mL | 4.2671 mL | 8.5342 mL | 10.6678 mL |
| 10 mM | 0.2134 mL | 1.0668 mL | 2.1336 mL | 4.2671 mL | 5.3339 mL |
| 50 mM | 0.0427 mL | 0.2134 mL | 0.4267 mL | 0.8534 mL | 1.0668 mL |
| 100 mM | 0.0213 mL | 0.1067 mL | 0.2134 mL | 0.4267 mL | 0.5334 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Salvianan
Catalog No.:BCN3545
CAS No.:862832-46-0
- IKK-3 Inhibitor
Catalog No.:BCC1643
CAS No.:862812-98-4
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
- Imatinib hydrochloride
Catalog No.:BCC1644
CAS No.:862366-25-4
- RA VII
Catalog No.:BCN3512
CAS No.:86229-97-2
- Valeriotriate B
Catalog No.:BCN6751
CAS No.:862255-64-9
- Mirodenafil
Catalog No.:BCC5254
CAS No.:862189-95-5
- Nandrolone undecylate
Catalog No.:BCC9090
CAS No.:862-89-5
- Anamorelin hydrochloride
Catalog No.:BCC1364
CAS No.:861998-00-7
- 7-Methoxy-3,4-dihydro-1-naphthalenylacetonitrile
Catalog No.:BCC8781
CAS No.:861960-34-1
- 2''-O-Beta-L-Galorientin
Catalog No.:BCN3804
CAS No.:861691-37-4
- A-740003
Catalog No.:BCC1322
CAS No.:861393-28-4
- Isoiridogermanal
Catalog No.:BCN7613
CAS No.:86293-25-6
- 10-Hydroxycanthin-6-one
Catalog No.:BCN3906
CAS No.:86293-41-6
- alpha-Amyrin acetate
Catalog No.:BCN4410
CAS No.:863-76-3
- Azilsartan Medoxomil
Catalog No.:BCC5021
CAS No.:863031-21-4
- Azilsartan medoxomil monopotassium
Catalog No.:BCC4089
CAS No.:863031-24-7
- Impurity C of Calcitriol
Catalog No.:BCC5384
CAS No.:86307-44-0
- Dasatinib monohydrate
Catalog No.:BCN2177
CAS No.:863127-77-9
- 8-epi-Chlorajapolide F
Catalog No.:BCN6426
CAS No.:863301-69-3
- PSI-6206
Catalog No.:BCC3609
CAS No.:863329-66-2
- Medetomidine
Catalog No.:BCC1736
CAS No.:86347-14-0
- Medetomidine HCl
Catalog No.:BCC4351
CAS No.:86347-15-1
- Cebranopadol
Catalog No.:BCC1467
CAS No.:863513-91-1
Tubulin polymerization-stimulating activity of Ganoderma triterpenoids.[Pubmed:28078535]
J Nat Med. 2017 Apr;71(2):457-462.
Tubulin polymerization is an important target for anticancer therapies. Even though the potential of Ganoderma triterpenoids against various cancer targets had been well documented, studies on their tubulin polymerization-stimulating activity are scarce. This study was conducted to evaluate the effect of Ganoderma triterpenoids on tubulin polymerization. A total of twenty-four compounds were investigated using an in vitro tubulin polymerization assay. Results showed that most of the studied triterpenoids exhibited microtuble-stabilizing activity to different degrees. Among the investigated compounds, ganoderic acid T-Q, ganoderiol F, ganoderic acid S, ganodermanontriol and Ganoderic acid TR were found to have the highest activities. A structure-activity relationship (SAR) analysis was performed. Extensive investigation of the SAR suggests the favorable structural features for the tubulin polymerization-stimulating activity of lanostane triterpenes. These findings would be helpful for further studies on the potential mechanisms of the anticancer activity of Ganoderma triterpenoids and give some indications on the design of tubulin-targeting anticancer agents.
Pharmacophore-based discovery of FXR-agonists. Part II: identification of bioactive triterpenes from Ganoderma lucidum.[Pubmed:22014750]
Bioorg Med Chem. 2011 Nov 15;19(22):6779-91.
The farnesoid X receptor (FXR) belonging to the metabolic subfamily of nuclear receptors is a ligand-induced transcriptional activator. Its central function is the physiological maintenance of bile acid homeostasis including the regulation of glucose and lipid metabolism. Accessible structural information about its ligand-binding domain renders FXR an attractive target for in silico approaches. Integrated to natural product research these computational tools assist to find novel bioactive compounds showing beneficial effects in prevention and treatment of, for example, the metabolic syndrome, dyslipidemia, atherosclerosis, and type 2 diabetes. Virtual screening experiments of our in-house Chinese Herbal Medicine database with structure-based pharmacophore models, previously generated and validated, revealed mainly lanostane-type triterpenes of the TCM fungus Ganoderma lucidum Karst. as putative FXR ligands. To verify the prediction of the in silico approach, two Ganoderma fruit body extracts and compounds isolated thereof were pharmacologically investigated. Pronounced FXR-inducing effects were observed for the extracts at a concentration of 100 mug/mL. Intriguingly, five lanostanes out of 25 secondary metabolites from G. lucidum, that is, ergosterol peroxide (2), lucidumol A (11), Ganoderic acid TR (12), ganodermanontriol (13), and ganoderiol F (14), dose-dependently induced FXR in the low micromolar range in a reporter gene assay. To rationalize the binding interactions, additional pharmacophore profiling and molecular docking studies were performed, which allowed establishing a first structure-activity relationship of the investigated triterpenes.


